Structure Hot Targets Published Data
Leveraging the cytotoxic potential of immune cells, particularly T cells, to augment anti-tumor responses has emerged as a promising approach in the management of cancer. Recently developed bispecific antibodies (BsAbs), notably bispecific T cell engagers (BiTEs), have accelerated the evolution of immunotherapeutic strategies by redirecting T cells toward malignant cells. Based on these, Creative Biolabs offers a specialized T Cell Engager (TCE)-based BsAb Design Service, facilitating investigation for our customers into solid tumor therapies.
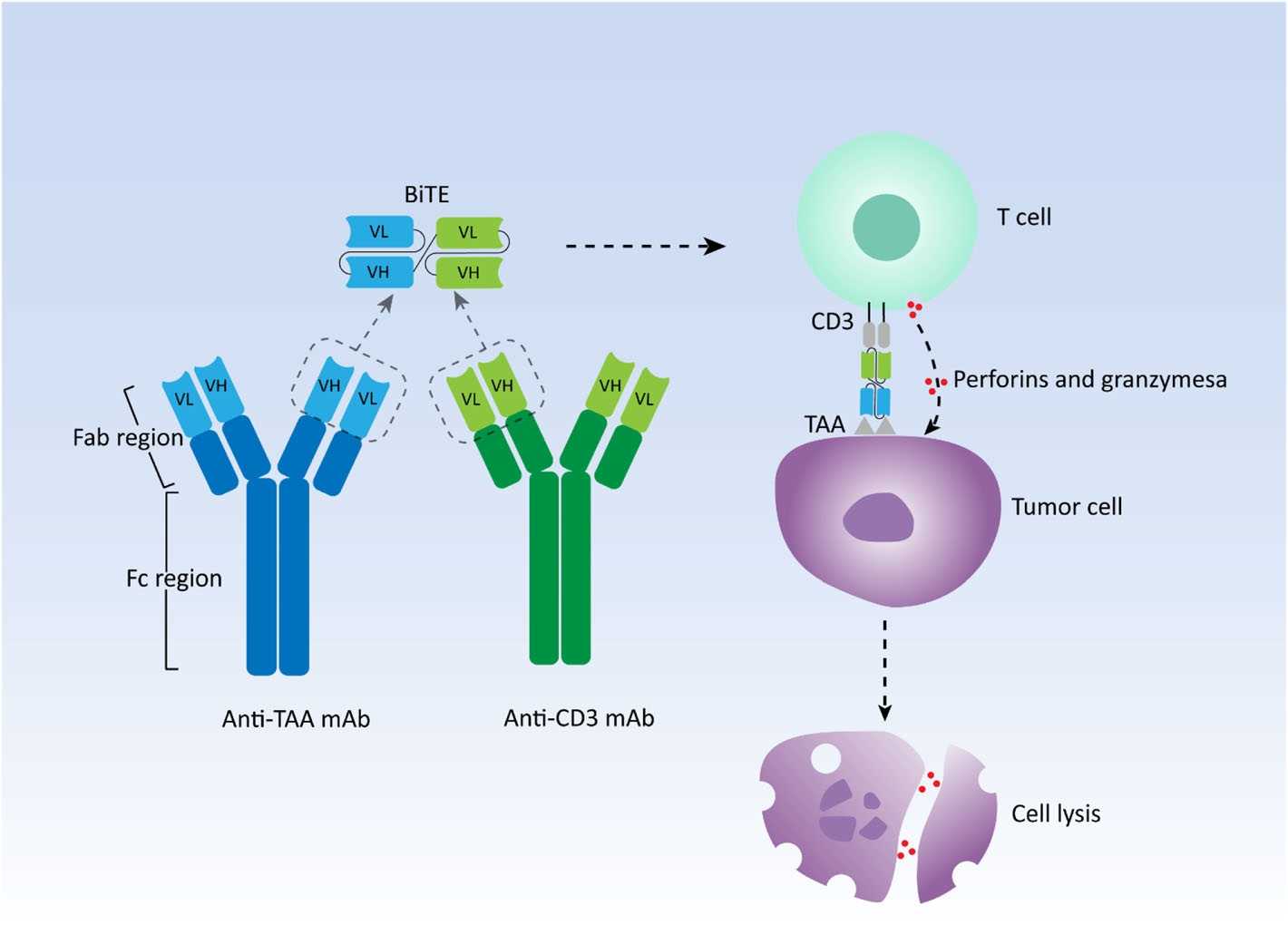 Fig.1 Schematic depiction of the architecture and functional mechanism of a prototypical bispecific T-cell engager.1, 3
Fig.1 Schematic depiction of the architecture and functional mechanism of a prototypical bispecific T-cell engager.1, 3
Structures of TCE-Based BsAbs Design
Leveraging the IgG architecture, BsAbs modeled on this framework exhibit a structure reminiscent of native immunoglobulins. The predominant approach to generating IgG-based BsAbs involves reassembling half-molecules obtained from distinct parental antibodies. Various strategies offered in our one-stop BsAb development platform—such as engineered orthogonal Fab interfaces and the knobs-into-holes (KIH) method, among others—have further refined the assembly of functional half-molecule constituents.
Based on their structural attributes, BsAbs are categorized into two main classes: those based on IgG and those constructed from variable fragment (Fv) elements, the latter typically possessing brief half-lives that require continuous administration. Alongside the BiTE format, platforms such as single-chain diabodies, dual-affinity retargeting antibodies (DARTs), and tandem diabodies (TandAbs) have been developed for the production of Fv-based BsAbs.
Hot Targets of TCE-Based BsAbs Design
TCE-based bispecific antibodies are engineered molecules integrating binding sites for both the T cell receptor (TCR) and tumor-associated or tumor-specific antigens, thereby conferring dual antigen specificity in a single construct. Among these, CD3-targeted antibodies are particularly prominent in cancer immunotherapy, as they reinforce TCR structural integrity and promote the signal transmission required for T cell activation. Antibodies against CD3 may either stimulate or dampen T cell signaling, influencing effector cell elimination or inducing a regulatory phenotype. Creative Biolabs offers a diverse portfolio of CD3 BsAbs.
Published Data
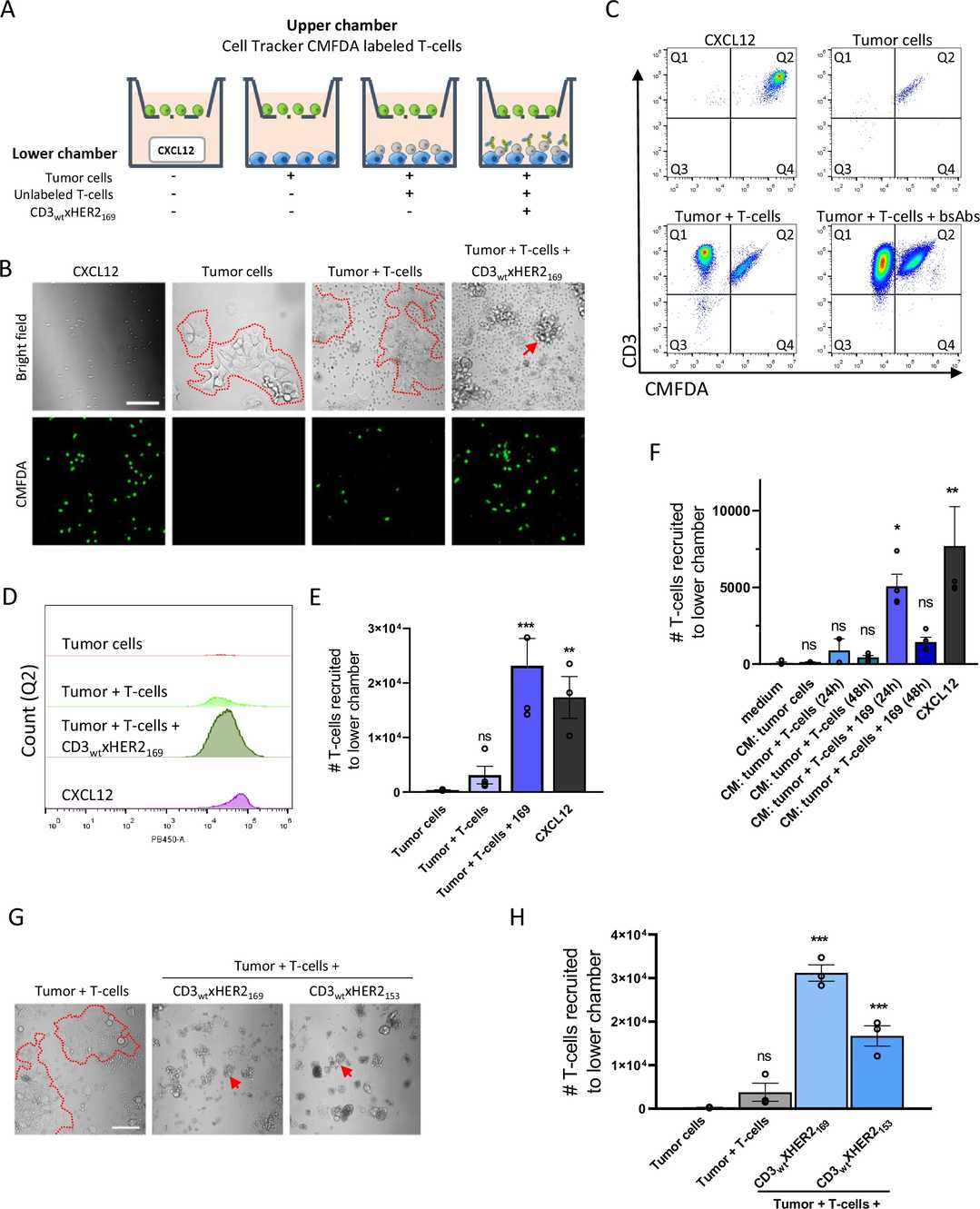 Fig.2 BsAb-facilitated T-cell and tumor cell interaction initiates chemotactic signaling for additional T-cell recruitment.2. 3
Fig.2 BsAb-facilitated T-cell and tumor cell interaction initiates chemotactic signaling for additional T-cell recruitment.2. 3
BsAbs targeting the CD3 T-cell receptor (TCR) and a tumor-associated antigen (TAA) can recruit T-cells to tumor sites, forming an immune synapse analogous to natural TCR-MHC/TAA interactions. Clinical investigations have demonstrated the efficacy of CD3xTAA BsAbs in treating non-Hodgkin's lymphoma and acute lymphoblastic leukemia. Researchers evaluated CD3xHER2 BsAbs in breast cancer tumoroid arrays with healthy donor-derived T-cells, finding that initial BsAb-mediated T-cell-tumor interactions induced chemotactic recruitment of additional T-cells, essential for effective tumoroid destruction.
Creative Biolabs boasts a highly skilled research team specializing in BsAbs, supported by advanced engineering platforms. We offer customized TCE-based BsAbs development services that encompass design, engineering, production, and comprehensive analysis.
References
-
Zhou, Shujie, et al. "The landscape of bispecific T cell engager in cancer treatment." Biomarker research 9.1 (2021): 38.
-
Liao, Chen-Yi, et al. "CD3-engaging bispecific antibodies trigger a paracrine regulated wave of T-cell recruitment for effective tumor killing." Communications Biology 7.1 (2024): 983.
-
Distributed under Open Access license CC BY 4.0, without modification.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Schematic depiction of the architecture and functional mechanism of a prototypical bispecific T-cell engager.1, 3
Fig.1 Schematic depiction of the architecture and functional mechanism of a prototypical bispecific T-cell engager.1, 3
 Fig.2 BsAb-facilitated T-cell and tumor cell interaction initiates chemotactic signaling for additional T-cell recruitment.2. 3
Fig.2 BsAb-facilitated T-cell and tumor cell interaction initiates chemotactic signaling for additional T-cell recruitment.2. 3


