Malaria is the most important infectious disease in the tropical and subtropical regions and continues to be a major global health problem. Prompt and accurate diagnosis is critical to the effective management of malaria. As a pioneer and the undisputed global leader in antibody discovery and manufacturing, Creative Biolabs offers in vitro diagnostic (IVD) antibody development services for diagnosis of a wide range of parasite infections including Malaria.
Introduction of Malaria
Malaria is a protozoan disease transmitted by Anopheles mosquitoes. Five species of the genus Plasmodium cause all malarial infections in human beings. The most serious and sometimes fatal type of malaria is caused by Plasmodium falciparum. The other human malaria species, P. vivax, P. ovale, P. malariae, and sometimes P. knowlesi can cause acute, severe illness but mortality rates are low. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause yellow skin, seizures, coma, or death. Since effective diagnosis reduces both complications and mortality from malaria, the need for sensitive laboratory diagnostic tools for confirmatory and differential diagnosis of Malaria is increasing.
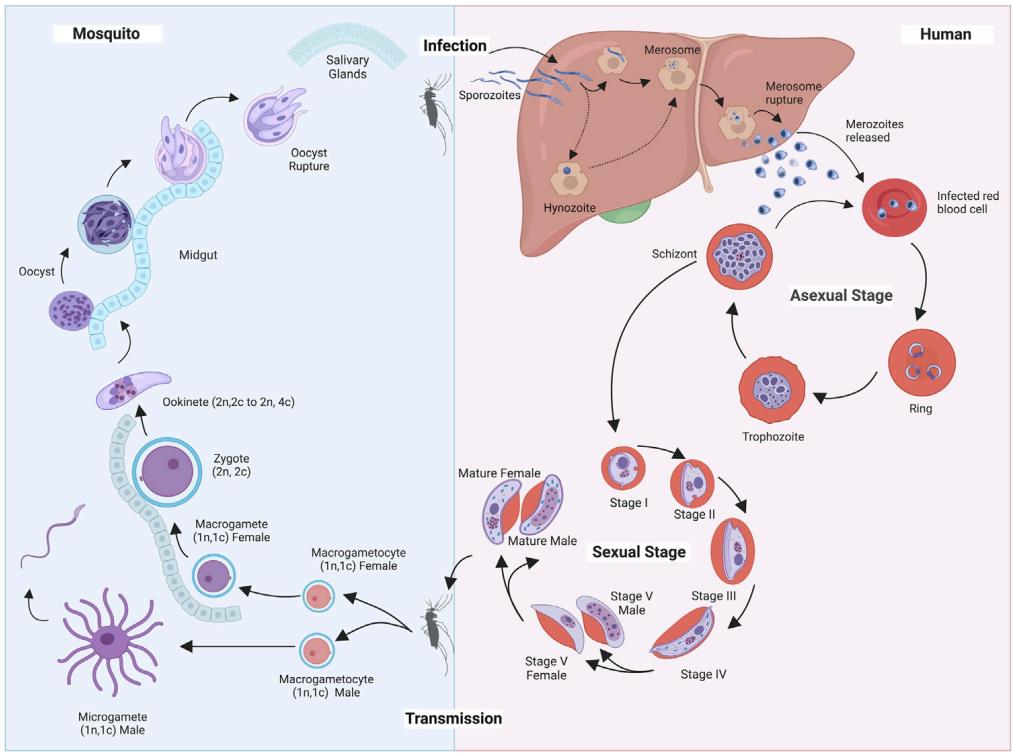 Fig.1 Lifecycle of Plasmodium falciparum in the human body and the anopheline mosquito.1
Fig.1 Lifecycle of Plasmodium falciparum in the human body and the anopheline mosquito.1
Laboratory Diagnosis of Malaria
The diagnosis of Malaria can be divided into clinical diagnosis and laboratory diagnosis. The former is based on the patient’s signs and symptoms, and on physical findings at the examination, while the latter uses different techniques, such as microscopic diagnosis by staining of blood smears, rapid diagnostic tests, and molecular diagnostic methods.
- Microscopy – Malaria is conventionally diagnosed by microscopic examination of stained blood films. Thick and thin blood films on a glass slide can be stained with Giemsa and are placed under the microscope for visualization of malaria parasites. It is widely used due to the simplicity, low cost, and its ability to identify the presence of parasites and assess the parasite density. However, the staining and interpretation processes are labor-intensive, time-consuming, and require considerable expertise and trained healthcare workers. Moreover, the shortcoming of microscopic examination is its relatively low sensitivity.
- Serological Tests – Diagnosis of malaria using serological methods is usually based on the detection of antibodies against asexual blood stage malaria parasites. Usually, specific antibodies against malaria can be produced within 2 wk of initial infection and persist for 3-6 months after parasite clearance. The most reliable and gold standard serological test in recent decades is the immunofluorescence antibody testing (IFA). It uses specific antigen to quantify the IgG and IgM antibodies in patient serum samples. IFA is simple and sensitive, but time-consuming. Moreover, it requires fluorescence microscopy and trained technicians.
- Rapid Diagnostic Tests (RDTs) – RDTs are based on the use of anti-malaria antibodies to detect malaria antigen in the blood. Most of the antibodies target a P. falciparum-specific protein, such as histidine-rich protein II (HRP-II) or lactate dehydrogenase (LDH). Some tests detect P. falciparum-specific and pan-specific antigens (aldolase or pan-malaria pLDH) and distinguish non-P. falciparum infections from mixed malaria infections. Many RDTs have been developed for detecting other malaria species, as well. Overall, RDTs appear a highly valuable, rapid diagnostic tool but they must be used in conjugation with other methods to confirm the results, characterize infection, and monitor treatment.
- Other Techniques – Recent developments in molecular biological technologies, e.g., PCR, loop-mediated isothermal amplification (LAMP), microarray, mass spectrometry (MS), and flow cytometric (FCM) assay techniques, have permitted extensive characterization of the malaria parasite and are generating new strategies for malaria diagnosis.
The quality of antibodies is of the utmost importance in the successful development of antibody-based IVD kits. With years of experience in the antibody development field, Creative Biolabs is capable of providing high-quality IVD antibody development services for the construction of different immunodiagnostic kits for clients both in the domestic and overseas. Contact us to discuss your specific requirements and experience the great value of our expert services.
Reference
- Chahine, Zeinab, and Karine G. Le Roch. "Decrypting the complexity of the human malaria parasite biology through systems biology approaches." Frontiers in systems biology 2 (2022): 940321. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.