Ultrasound molecular imaging has been developed in the past two decades with the goal of non-invasively imaging disease phenotypes on a cellular level not depicted on anatomic imaging. Such techniques already play a role in pre-clinical research for the assessment of disease mechanisms and drug effects and are thought to contribute to earlier diagnosis of disease in the future, assessment of therapeutic effects and patient-tailored therapy in the clinical field. Creative Biolabs is an integrated antibody development and molecular imaging research services provider to the institute and companies. We possess advanced and comprehensive molecular imaging techniques to accelerate your drug/device discovery and development stage research. We provide ultrasound-targeted nanobubble development services for in vivo imaging research use.
Introduction of Ultrasound Contrast Agents
Ultrasound is a safe, fast, and inexpensive technique widely used in diagnosis. This imaging technique makes use of sound waves (typically with frequencies in the range of 1 to 40 MHz) which are reflected differently by different organs and tissues. When compared to other imaging modalities such as optical imaging and single-photon emission computerized tomography (SPECT) imaging, the image quality in ultrasound is often inferior as blood is a poor scatterer of ultrasound waves. Microbubbles are generally used to enhance contrast in ultrasound imaging due to their superior scattering properties and their dynamic response to the application of an ultrasonic field. Recently, many studies focused on sdAb-based ultrasound imaging have been reported.
 Fig.1 SPECT.Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Fig.1 SPECT.Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Targeting Strategies for Ultrasound Molecular Imaging
In general, there are two strategies for targeting of ultrasound contrast agents: modification of the shell surface, and conjugation of a targeting ligand on the contrast agent shell surface. Incorporation of phosphatidylserine into the microbubble shell results in a highly negative surface charge and in activation and surface attachment of complement fragments, which in turn mediate attachment of microbubbles to activated leukocytes within inflamed tissue. In a more specific strategy to targeting, the conjugation of ligands such as sdAb to the microbubble surface is used. Conjugation can be accomplished either directly to the microbubble shell or more often to a PEG spacer arm to project the binder away from the microbubble surface and, therefore, enhance ligand-target interaction.
Due to the micrometer size, the proteins targeted should be located in the vasculature of the tumor. For instance, sdAbs targeting the vascular cell adhesion protein 1 (VCAM1) were developed for microbubble targeting, as this protein is involved in melanoma cell adhesion to the endothelium. Anti-VCAM1 sdAbs were coupled to lipid microbubbles by biotinylation and injected intravenously to mice bearing MC38 tumor xenografts. The signals gained from the sdAb coupled microbubbles were found to be enhanced compared to the control microbubbles.
Although only the vasculature can be probed with this technique, the application of sdAb-targeted microbubbles is beneficial as it can provide safe, fast and inexpensive scanning together with relatively high-quality images.
Application Fields
- Molecular imaging of thrombosis
- Molecular imaging of atherosclerosis
- Molecular imaging of microvascular inflammation
- Molecular imaging in oncology
- ……
Compared with other imaging methods, a distinct advantage of ultrasound is its wide availability and low cost. Thus, from a technical and economical point of view, ultrasound is well suited for screening of large populations for a particular disease phenotype. If you are interested in our customized ultrasound-targeted nanobubble development services, please directly contact us for more information.
Published Data
1. A New Targeting Microbubble for Ultrasound Molecular Imaging
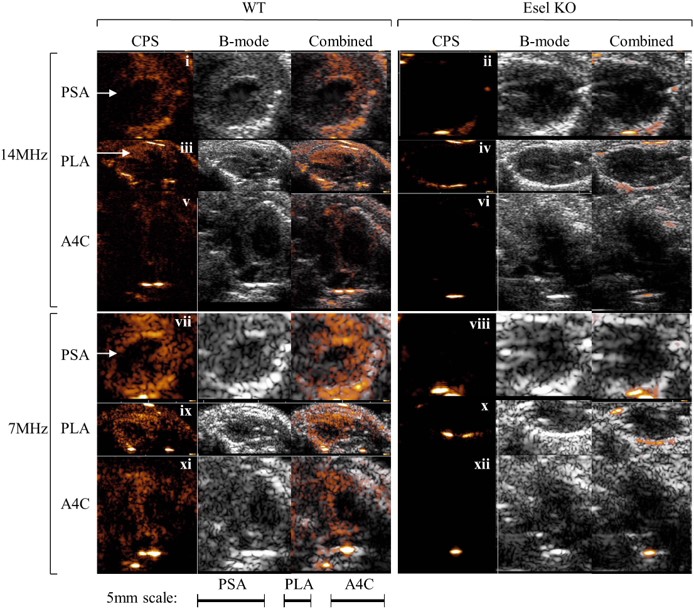 Fig.2 Real-time ultrasound molecular imaging of E-selectin (Esel) expression in the mouse heart.1,3
Fig.2 Real-time ultrasound molecular imaging of E-selectin (Esel) expression in the mouse heart.1,3
This study developed and evaluated a targeting microbubble for ultrasound molecular imaging using maleimide-thiol conjugation chemistry. Microbubbles, composed of generic (non-targeting) components, were conjugated with anti-E-selectin F(ab’)2 to create E-selectin-targeting microbubbles. These targeting bubbles exhibited high specificity for E-selectin both in vitro and in vivo, with minimal non-specific retention in non-reticuloendothelial tissues (heart, kidneys, cremaster) showing inflammation. Using a clinical ultrasound scanner, the microbubbles allowed for real-time imaging of E-selectin expression in the inflamed hearts and kidneys of mice. Acoustic signal intensity correlated strongly with E-selectin expression levels (|r|≥0.8), demonstrating high non-invasive molecular quantification. The maleimide-thiol conjugation-based targeting microbubbles show potential for clinical applications due to their low non-specific bubble retention in tissues, effectiveness in real-time imaging, and high acoustic quantification of molecular targets.
2. Biomimetic Nanobubbles for Targeted Ultrasound Molecular Imaging of Triple-Negative Breast Cancer
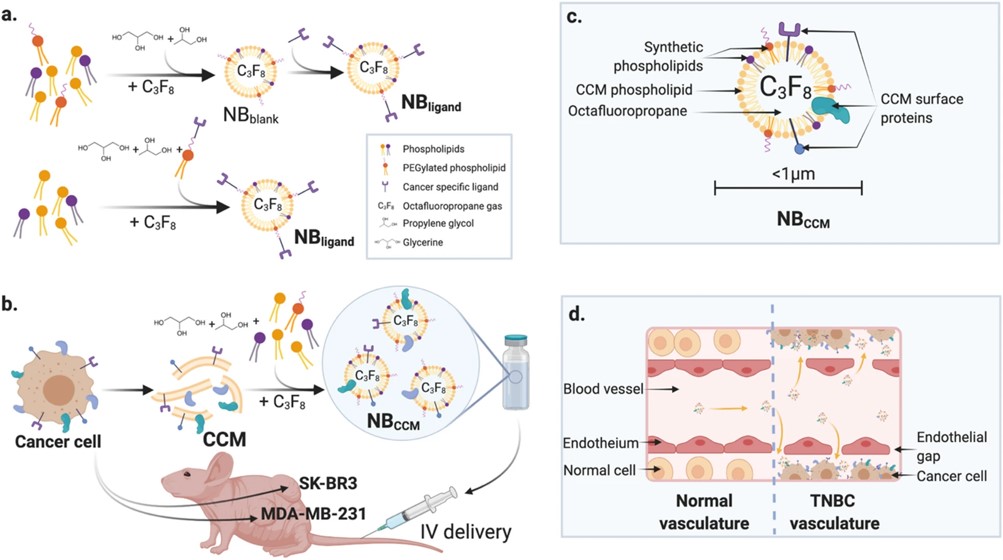 Fig.3 Tumor-targeted NBs.2,3
Fig.3 Tumor-targeted NBs.2,3
In this study, researchers engineered the first nanobubbles (NBs) based on triple-negative breast cancer (TNBC) cell membranes (NBCCM) as a targeted diagnostic agent, exploiting the homotypic recognition of cancer cells. Using microfluidic technology, they synthesized NBCCM by leveraging the self-assembly properties of cell membranes in aqueous solutions. The optimal NBCCM, with a hydrodynamic diameter of 683 ± 162 nm, exhibited long-lasting ultrasound contrast enhancements and strong homotypic affinity in vitro. In vivo, NBCCM showed significantly greater extravasation and retention in a TNBC mouse model than non-targeted NBs, as demonstrated by enhanced ultrasound molecular imaging. Time-intensity plots revealed a 2.1-fold and 3.6-fold increase in peak intensity and area under the curve, respectively (P < 0.01). Immunofluorescence further confirmed NBCCM presence within the tumor microenvironment. This approach, overcoming challenges in universal cancer biomarker identification, could enable TNBC targeting, improved diagnosis and potentially gene/drug targeted delivery, and to image other cancers using biomimetic NBs from their respective cancer cell membranes.
References
- Yeh, James Shue-Min, et al. "A targeting microbubble for ultrasound molecular imaging." PloS one 10.7 (2015): e0129681.
- Jugniot, Natacha, et al. "Biomimetic nanobubbles for triple-negative breast cancer targeted ultrasound molecular imaging." Journal of Nanobiotechnology 20.1 (2022): 267.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.