Intestinal Tissue Exosome Research and Application
Overview Services Features FAQs
The study of intestinal tissue-derived exosomes and their cargo is necessary to understand the intestinal physiological environment and crosstalk with remote organs. Creative Biolabs provides services for the isolation of high-quality exosomes from intestinal tissue and downstream studies.
Intestinal Tissue Exosome Overview
-
The intestine is the biggest organ consisting of a single layer of cells with the largest surface area, and it is also the central regulator of the body's energy homeostasis. As the primary organ of nutrient sensing and absorption, the intestine contains not only a variety of changing dietary components and their digestive secretions but also a wide variety of flora and their metabolites, which influence the secretory regulation of the intestine.
-
Intestinal tissue exosomes mediate the transfer of substances and information between the intestine and remote organs as a communication link and play a variety of functions in healthy and diseased intestinal homeostasis, including intestinal barrier integrity, intestinal epithelial wound healing, immunogenesis, and intestinal microbiota establishment. Therefore, it is beneficial to explore intestinal tissue exosomes to understand the secretory regulatory functions of the intestinal tract and the mechanisms by which it coordinates with multiple organs to maintain the metabolic homeostasis of the body.
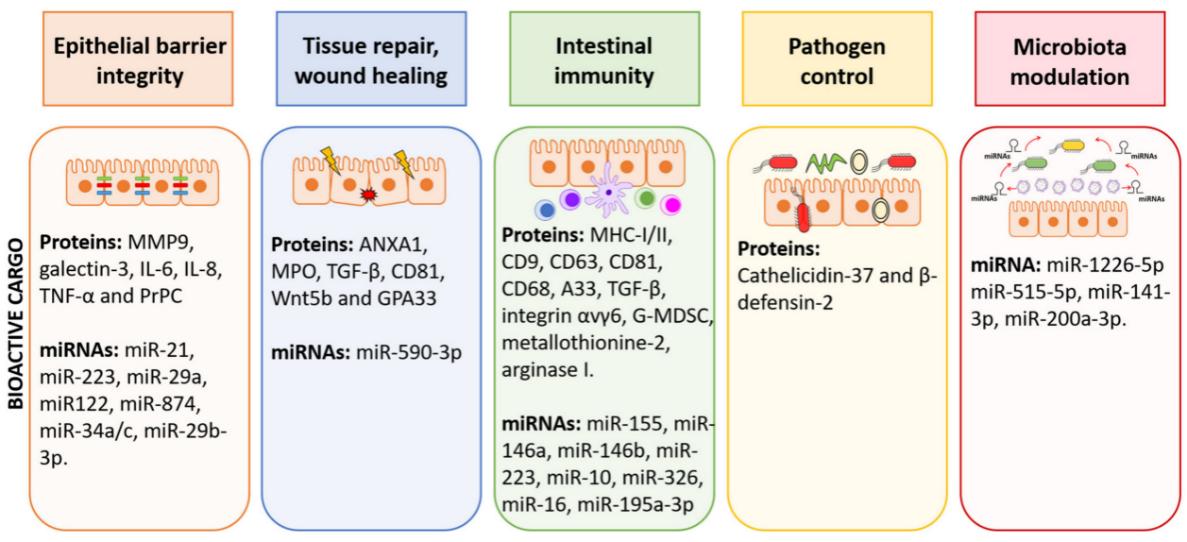 Fig.1 The function of EVs in the intestinal environment.1
Fig.1 The function of EVs in the intestinal environment.1
Our Services
At Intestinal Tissue Exosome Research and Application, we specialize in providing comprehensive research services aimed at enhancing the understanding and utility of exosomes derived from intestinal tissues. Our offerings include exosome isolation and characterization, functional assays to investigate their roles in intercellular communication, and advanced analytical techniques to profile their molecular contents. We support various projects, ranging from basic research to the development of innovative applications in fields such as biotechnology.
Intestinal Tissue Exosome Features
The sources of intestinal tissue exosomes include intestinal epithelial cells, immune cells, and intestinal microorganisms. Their involvement in the regulatory function of the intestinal ecosystem and its mechanisms in multiple modes are directions for the study of intestinal tissue exosomes, such as
-
Transmitting signaling molecules: Exosomes contain a variety of signaling molecules affecting the function of the intestinal ecosystem. For example, miR-125a/b from intestinal fibroblast-derived exosomes in the short bowel syndrome model has been shown to regulate GLP-2 (glucagon-like peptide-2)-mediated proliferation and apoptosis of intestinal epithelial cells. In this study, researchers extracted exosomes from residual jejunal tissue of GLP-2-treated and control rat models of the disease, as well as miRNA sequencing and qRT-PCR to screen for differentially expressed miRNAs involved in the proliferation and apoptosis of intestinal epithelial cells. Meanwhile, based on the predicted results of miRNA target genes in the raw letter analysis, the downstream pathways of miRNAs were further explored by miRNA function recovery experiments, target gene knockdown, and overexpression experiments to elucidate new mechanisms of intestinal tissue exosomes mediating genetic exchange in the intestinal microenvironment. In addition, the role and mechanism of intestinal tissue exosomes, especially from intestinal epithelial cells and immune cells, in promoting intestinal repair and healing by altering the expression of local growth factors and transcription factors in the intestine is also worth investigating.
-
Regulation of microbial communities: intestinal tissues secrete exosomes in a polarized manner and are the main source of delivering miRNAs that can break through the natural mucus barrier of the intestine itself and affect the growth and metabolism of intestinal microbes. These intestinal exosome-mediated miRNA interactions with the flora are able to regulate the structure and function of the intestinal microbial community and maintain the balance of the intestinal ecosystem. For example, miR-1226-5p promotes Escherichia coil growth while miR142a-3p promotes Lactobacillus reuteri growth. In addition, intestinal tissue exosome-loaded proteins and lipids also contribute to regulating the intestinal microbiota by affecting carbon and nitrogen sources in the gut, respectively.
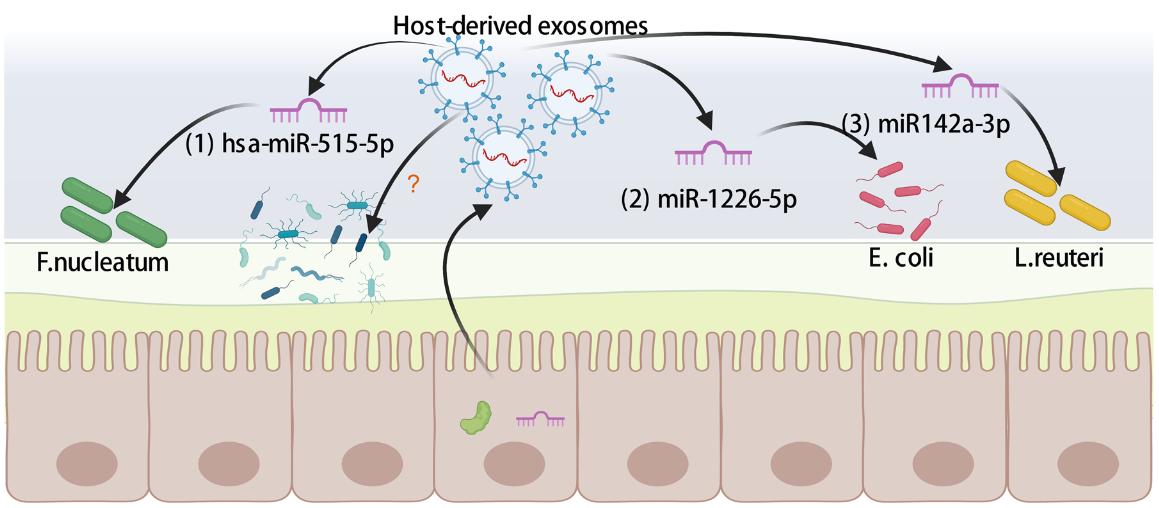 Fig.2 Gut-derived exosomal shaping gut microbiota.2
Fig.2 Gut-derived exosomal shaping gut microbiota.2
-
Enhancing the immune response: exosomes enhance the intestinal immune response by activating immune cells to improve intestinal defenses, thereby preventing and alleviating enteritis and infection. It has been found that exosomes secreted by intestinal neutrophils in inflammatory states contain large amounts of matrix metalloproteinases-9 that disrupt the structural support of the intestinal barrier by cleaving the desmoglein-2 involved in intercellular adhesion, blocking the connection between the junction protein and the actin cytoskeleton. In contrast, the site of intestinal inflammation recruits a large number of macrophages, which release exosomes containing galectin-3 that act and increase the stability of desmoglein-2 at the intercellular adhesion site, promoting the integrity of the intestinal barrier. Moreover, these macrophages can phagocytose exosomes secreted by intestinal neutrophils, induce the TGF-β1 release, and attenuate the local inflammatory response.
-
Involvement in systemic metabolic regulation: Intestinal tissue exosomes are also capable of delivering a variety of metabolic substances involved in the regulation of systemic metabolism. This is mainly due to the secretion of exosomes by intestinal tissue cells into the submucosa, which in turn reaches distal organs or tissues through the submucosal reflux system, enabling remote regulation of metabolism. A high-fat diet has been reported to shape the lipid profile of intestinal tissue exosomes and regulate communication in the liver/intestinal axis. This was achieved mainly by performing LC-MS-based exosomal lipidomic analysis, array-based exosomal cytokine analysis, and metabolic monitoring.
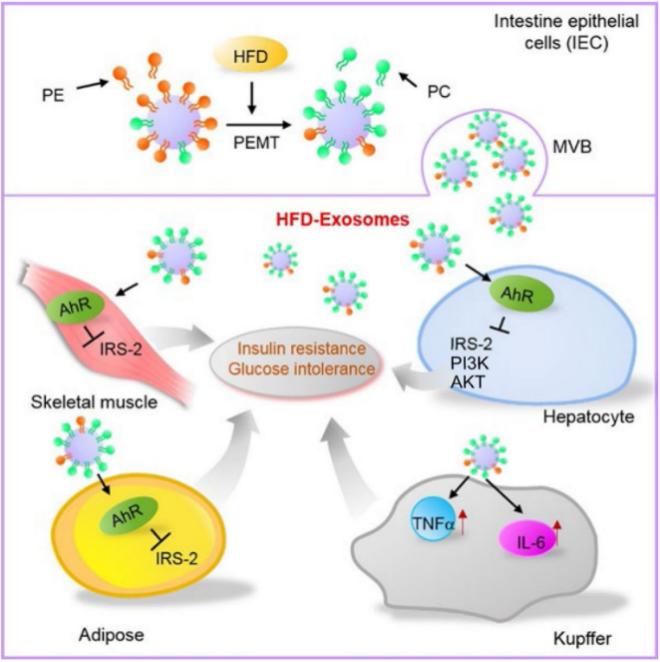 Fig.3 Intestinal epithelial cell exosomes from high-fat fed mice.3
Fig.3 Intestinal epithelial cell exosomes from high-fat fed mice.3
Creative Biolabs provides services including isolation, identification, functional assays, and mechanistic research of intestinal tissue exosomes, which assist in a more realistic understanding of their donor intestinal tissue status. Please contact us with your interest.
FAQs
Q: What types of intestinal tissues do you accept for exosome analysis?
A: We accept a wide range of intestinal tissues, including those from different animal species, and can tailor our protocols to accommodate specific requirements related to the tissue source and research goals.
Q: How do you ensure the quality and integrity of the exosomes isolated from intestinal tissues?
A: Our isolation protocols incorporate stringent quality control measures, including the use of validated characterization techniques such as NTA to assess size and concentration.
Q: Can you customize your services for specific experimental needs?
A: Yes, we work closely with our clients to customize our services based on the specific research objectives, whether that involves specialized exosome analysis or the development of unique assays.
Q: What types of assays do you offer to investigate the functions of intestinal exosomes?
A: We provide a variety of assays, including cell uptake studies, signaling pathway analyses, and functional assays to evaluate the role of intestinal exosomes in mediating communication between intestinal cells and other cell types.
References
-
Diaz-Garrido, Natalia, et al. "Cell-to-cell communication by host-released extracellular vesicles in the gut: implications in health and disease." International Journal of Molecular Sciences 22.4 (2021): 2213. Distributed under Open Access license CC BY 4.0. The image was modified by revising the title.
-
Zhang, Chenguang, et al. "An overview of host‐derived molecules that interact with gut microbiota." Imeta 2.2 (2023): e88. Distributed under Open Access license CC BY 4.0. The image was modified by revising the title.
-
Kumar, Anil, et al. "High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance." Nature communications 12.1 (2021): 213. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only Part D of the original image and by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 The function of EVs in the intestinal environment.1
Fig.1 The function of EVs in the intestinal environment.1
 Fig.2 Gut-derived exosomal shaping gut microbiota.2
Fig.2 Gut-derived exosomal shaping gut microbiota.2
 Fig.3 Intestinal epithelial cell exosomes from high-fat fed mice.3
Fig.3 Intestinal epithelial cell exosomes from high-fat fed mice.3









