Kidney Tissue Exosome Research and Application
Overview Services Features FAQs
Creative Biolabs summarizes the classical research ideas of renal tissue exosomes, including functional mechanism study, targeting key tissue exosome molecules, clinical significance validation, and in vitro functional validation.
Overview
Tissue exosomes are the true functioning exosomes in diseased tissues in vivo. As exosomes of various cellular origins are also pooled in the tissue microenvironment, tissue exosomes are authentic and abundant compared to cell lineage-derived exosomes, and exclusive and innovative compared to humoral exosomes.
Kidney tissue exosomes have a place in the study of tissue exosomes. Each kidney consists of more than 4 million nephron units. Each nephron unit includes three parts: glomerulus, renal capsule, and tubules. The sources of kidney tissue exosomes include renal tissue, tubular tissue, and renal tumor tissue, and are involved in diseases such as chronic kidney disease, acute kidney injury, chronic kidney injury, and kidney cancer.
Services We offer
We have successfully launched services for kidney tissue exosome research and applications, accumulating extensive project experience in isolating high-purity exosomes from kidney tissue. We also offer characterization, content analysis, and application studies.
Kidney Tissue Exosomes
-
Functional Mechanism Investigations
A study explored how exosomes from renal tubules contribute to renal fibrosis. It found increased exosome secretion in renal injury, indicated by CD63 expression and confirmed through electron microscopy. Proximal tubular epithelial cells were identified as the primary source of these exosomes. In vitro and in vivo experiments demonstrated that these exosomes activate fibroblasts and promote renal fibrosis by transporting Sonic hedgehog ligands.
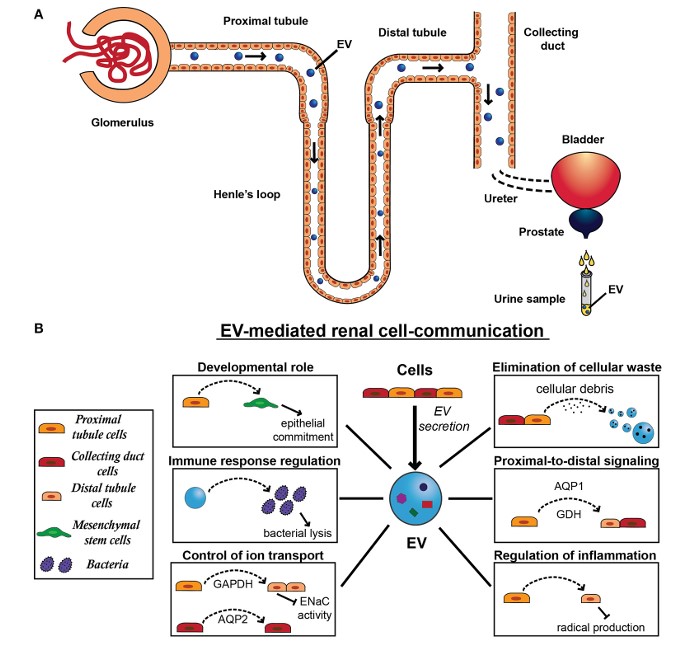 Fig.1 Exosome secretion and physiological function in the kidney.1
Fig.1 Exosome secretion and physiological function in the kidney.1
-
Targeting Tissue Exosome Key Molecules and Validating Clinical Significance
It is also valuable to target key exosomal molecules and explore their major contributing sites and clinical significance in the disease. Researchers identified miR-19b-3p through global miRNA profiling of exosomal contents in an AKI model, comparing diseased and normal kidney tissues. Exosomes isolated from tubular kidney tissues showed a significantly higher level of miR-19b-3p in diseased states compared to control groups, indicating selective loading of miRNAs into renal secretory exosomes, particularly from renal proximal tubule epithelial cells. This loading led to M1 macrophage activation and inflammation. Additionally, the clinical relevance of elevated exosomal miR-19b-3p in urine samples from diabetic nephropathy was examined, correlating with the severity of tubulointerstitial inflammation.
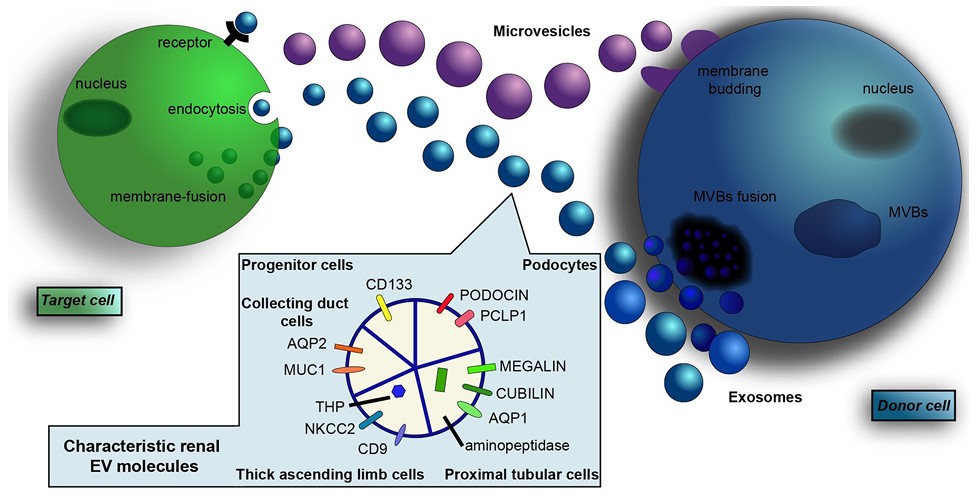 Fig.2 Renal-derived exosomes.1
Fig.2 Renal-derived exosomes.1
-
Omics Analysis and Functional Validation
After speculating the biological functions of tissue exosomes and screening their key functional molecules, setting up in vitro experiments to validate the functions of cell- and tissue-derived exosomes facilitates the identification of reliable disease-related exosome markers. For example, protein profiling of exosomes from renal cell carcinoma and normal kidney tissue showed significant enrichment of Azurocidin 1, correlating with clinical staging and grading confirmed through differential analysis and western blot. In vitro experiments demonstrated that exosomes from renal cell carcinoma disrupted the morphology of vascular endothelial cells in an Azurocidin 1-dependent manner, highlighting the potential of Azurocidin 1 as a marker for early renal cell carcinoma.
Comprehensive omics analysis and functional exploration of renal tissue-derived exosomes provide exciting strategies for understanding the physiological functions of exosomes in kidney-related diseases. Creative Biolabs provides services for kidney tissue exosome isolation, omics profiling, and functional studies to deeply explore the significant advantages of tissue exosomes in developing markers and interpreting function. Please contact us to learn more.
FAQs
Q: How do kidney tissue exosomes differ from those derived from other tissues in terms of composition and function?
A: Kidney tissue exosomes have a unique molecular signature that reflects the physiological and pathological state of the kidneys. They contain specific contents species that play crucial roles in kidney function and pathology, enabling targeted research and therapeutic strategies that are specific to renal conditions.
Q: How is the function of renal tissue exosomes in intercellular communication being studied?
A: Research is being conducted to understand how kidney tissue exosomes facilitate communication between renal cells and the extracellular environment. Studies focus on identifying the signaling molecules contained within exosomes and their influence on processes such as inflammation, fibrosis, and regeneration in the kidneys.
Q: Which novel approaches are being applied to the investigation of exosomes from kidney tissue?
A: Cutting-edge techniques such as mass spectrometry and proteomics are being employed to analyze the content of kidney tissue exosomes. Additionally, advanced imaging techniques, such as super-resolution microscopy, are enhancing our understanding of exosome dynamics and their interactions with target cells.
Q: What future research directions are being considered for kidney tissue exosomes?
A: Future research may focus on understanding the full spectrum of functions of kidney tissue exosomes, including their role in kidney stone formation and transplant rejection. Additionally, efforts are being made to explore their potential in regenerative medicine and tissue engineering, as well as their use in developing novel therapeutic agents.
Q: How do kidney tissue exosomes compare to traditional methods of studying kidney function?
A: Kidney tissue exosomes offer a non-invasive means to study renal function and pathology, which contrasts with traditional methods such as biopsies or blood tests. They provide insights into cellular communication and molecular pathways in a dynamic manner, making them valuable tools for early detection and real-time monitoring of kidney conditions.
Reference
-
Pomatto, Margherita AC, et al. "Extracellular vesicles in renal pathophysiology." Frontiers in molecular biosciences 4 (2017): 37. Under open access license CC BY 4.0. The image was modified by revising the titles.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Exosome secretion and physiological function in the kidney.1
Fig.1 Exosome secretion and physiological function in the kidney.1
 Fig.2 Renal-derived exosomes.1
Fig.2 Renal-derived exosomes.1









