Bone Tissue Exosome Research & Application
Introduction Research Direction Services FAQs
Unlocking the Secrets of Bone and Cartilage: The Power of Bone Tissue-Derived Exosomes
The dynamic processes of bone remodeling and cartilage maintenance, has long captivated scientific inquiry. Orthopedic diseases like rheumatoid arthritis, osteoarthritis, femoral head necrosis, and osteoporosis present significant clinical challenges, and our understanding of their pathogenesis remains incomplete. Traditionally, research has relied on exosomes isolated from the supernatant of in vitro cell cultures or from systemic biofluids like plasma. However, these methods often fail to capture the nuanced and localized intercellular communication that occurs within bone and cartilage tissues.
At Creative Biolabs, we believe that to truly understand the complex microenvironment of these tissues, we must go directly to the source. That is why we are at the forefront of research into tissue-derived exosomes, specifically those isolated from bone and cartilage. Unlike exosomes found in cell cultures or diluted in systemic fluids, these tissue-resident exosomes are secreted by the very cells that constitute the tissue—osteocytes, osteoblasts, osteoclasts in bone, and chondrocytes in cartilage—providing an unparalleled, authentic snapshot of the local disease state. By analyzing the unique molecular cargo of these exosomes, we can gain a deeper understanding of disease mechanisms and uncover more specific and accurate diagnostic and therapeutic markers.
Ready to innovate? Partner with us to explore the full potential exosomes.
Groundbreaking Findings in Bone and Cartilage Research
The study of tissue-derived exosomes is revealing their profound involvement in both the physiological function and the pathogenesis of various orthopedic diseases.
Recent studies have showcased the crucial roles of bone and cartilage exosomes, providing new avenues for understanding and treating orthopedic diseases.
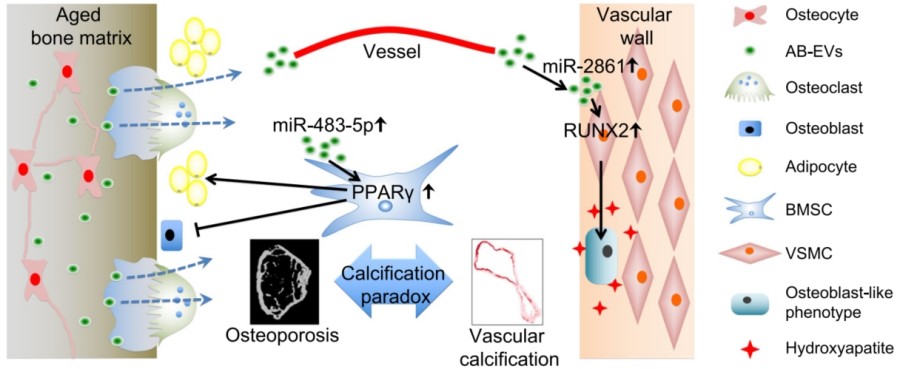 Fig.1 Schematic diagram showing the role of AB- Fig.2 EVs as a messenger for calcification paradox by favoring BMSC adipogenesis and VSMC calcification.1,3
Fig.1 Schematic diagram showing the role of AB- Fig.2 EVs as a messenger for calcification paradox by favoring BMSC adipogenesis and VSMC calcification.1,3
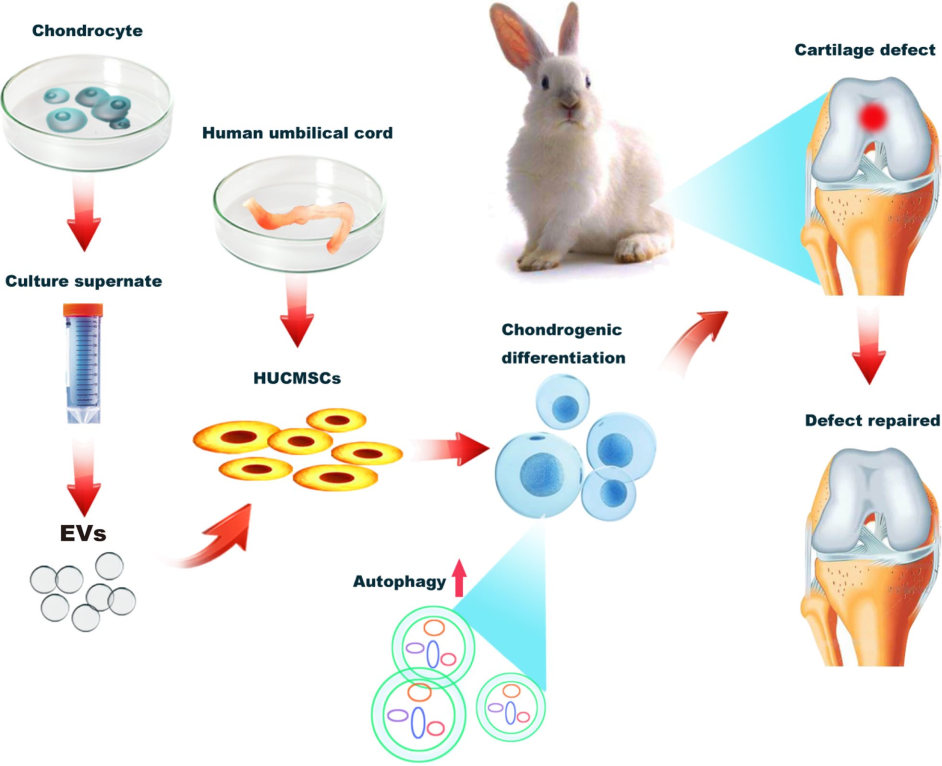 Fig.3 The graphic illustration for C-EVs promoting chondrogenic differentiation of HUCMSCs.2,3
Fig.3 The graphic illustration for C-EVs promoting chondrogenic differentiation of HUCMSCs.2,3
|
Tissue Source of Exosomes
|
Key Findings & Mechanisms
|
Significance for Your Research
|
|
Bone matrix
|
Aged bone matrix-derived exosomes (EVs) act as messengers, transferring specific microRNAs (e.g., miR-483-5p, miR-2861) from bone to the vascular wall. These EVs simultaneously promote fat accumulation in bone and arterial wall calcification.
|
Uncovers a novel mechanism behind the "calcification paradox" of osteoporosis and vascular disease, offering new therapeutic targets beyond traditional treatments.
|
|
Cartilage
|
Exosomes isolated from cartilage tissue can safely promote the proliferation and differentiation of stem cells (HUCMSCs). This process, mediated by activating autophagy, occurs without the risk of cartilage hypertrophy, a common side effect of other therapies.
|
Provides a safer, more effective strategy for cartilage repair and regeneration, offering a new path forward for treating conditions like osteoarthritis.
|
These findings underscore the power of directly analyzing tissue-derived exosomes. They are not merely passive indicators; they are active agents driving disease progression and holding the key to regenerative processes.
Need a partner for your research? Let's discuss your project.
Future Directions and Applications
The burgeoning field of bone and cartilage tissue exosome research points toward several transformative applications that we are uniquely positioned to help our clients explore.
Elucidating Exosome-Based Disease Mechanisms
The ability to analyze the specific proteomic, lipidomic, and nucleic acid cargo within these tissue-specific exosomes offers an unparalleled opportunity to dissect the intricate molecular mechanisms underlying orthopedic diseases. By identifying key signaling pathways and cellular interactions, we can pinpoint the drivers of disease progression and identify new therapeutic targets.
Developing Precision Liquid Biopsies
Exosomes in systemic circulation are often diluted and mixed, obscuring tissue-specific signals. By first identifying the unique molecular signatures of exosomes from diseased bone and cartilage tissue, we can then develop highly specific liquid biopsy markers. This approach would allow for non-invasive, early diagnosis, disease staging, and the monitoring of therapeutic responses, moving beyond general indicators to pinpoint disease activity at its source.
Unlock your project's potential. Discuss your specific needs with our specialists.
Partner with Creative Biolabs
At Creative Biolabs, our over two decades of experience in molecular and cellular biology have positioned us at the forefront of this emerging field. We understand the unique complexities of working with bone and cartilage tissues and have developed specialized platforms to support every stage of your research. Our professional team of leading experts is dedicated to translating cutting-edge discoveries into tangible solutions for orthopedic health.
We offer a comprehensive, one-stop suite of services tailored specifically for bone and cartilage tissue exosome research:
Tissue exosome extraction and identification services
Tissue exosome profiling service
Combined Research Services
Tissue exosome in vivo and in vitro function research services
We employ advanced and optimized methods to ensure high-quality, pure, and intact exosome preparations from a variety of tissue sources. Our state-of-the-art characterization services provide precise insights into exosome size, morphology, and surface markers.
Our in-depth analysis services, including proteomic, RNA sequencing, lipidomic, and metabolomic profiling, allow you to uncover the full bioactive cargo within your tissue exosomes.
Combined Research Services
To provide the most comprehensive picture, we offer body fluid exosome and tissue exosome combined research service, tissue exosome and individual cell combined transcriptomics and proteomics research services. This approach allows you to connect local tissue events with systemic changes and cellular function.
We provide robust experimental systems, including advanced animal models and exosome labeling technologies, to evaluate the mechanistic pathways of bone and cartilage tissue exosomes in vivo and in vitro.
We invite you to contact us to discuss your specific research ideas. Our professional scientific team is eager to collaborate with you to explore the mysteries of bone and cartilage tissue exosomes.
FAQs
Q: Why are exosomes from bone and cartilage tissue more informative than those from blood or cell culture?
A: Tissue-derived exosomes are primarily secreted by the cells within the local microenvironment of bone and cartilage. This means their molecular cargo—proteins, lipids, and nucleic acids—directly reflects the specific pathological changes occurring in that particular region. Exosomes from blood are a mixed population from various tissues, and even those from cell cultures may not fully replicate the complex intercellular signaling that takes place in vivo. For these reasons, tissue exosomes provide a more authentic and exclusive representation of the localized disease state.
Q: What are the main challenges in isolating exosomes from bone and cartilage, and how do you overcome them?
A: The primary challenge is the dense and complex extracellular matrix of these tissues. This structure makes it difficult to extract exosomes without causing cellular damage or contamination. Our optimized isolation methods, which include specific enzymatic digestion followed by advanced ultracentrifugation, are designed to carefully liberate exosomes while preserving their integrity and purity. We also utilize rigorous characterization techniques to validate the quality of every isolation.
Q: How can Creative Biolabs help me with my bone and cartilage tissue exosome research?
A: We offer comprehensive, end-to-end services. From initial tissue exosome isolation and meticulous characterization to in-depth profiling and functional studies in vitro and in vivo, our expertise and state-of-the-art platforms are designed to provide precise insights and accelerate your project from discovery to validation. We can help you identify novel biomarkers, explore disease mechanisms, and even lay the groundwork for new therapeutic approaches. If you have a research project in mind or want to learn more about how tissue-derived exosomes can advance your work, please don't hesitate to get in touch. We look forward to hearing from you.
Let's discuss how our services can provide the solutions you need. Inquire today.
References
-
Wang, Zhen-Xing, et al. "Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox." Nature communications 13.1 (2022): 1453. https://doi.org/10.1038/s41467-022-29191-x
-
Ma, Ke, et al. "Articular chondrocyte-derived extracellular vesicles promote cartilage differentiation of human umbilical cord mesenchymal stem cells by activation of autophagy." Journal of Nanobiotechnology 18.1 (2020): 163. https://doi.org/10.1186/s12951-020-00708-0
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:


 Fig.1 Schematic diagram showing the role of AB- Fig.2 EVs as a messenger for calcification paradox by favoring BMSC adipogenesis and VSMC calcification.1,3
Fig.1 Schematic diagram showing the role of AB- Fig.2 EVs as a messenger for calcification paradox by favoring BMSC adipogenesis and VSMC calcification.1,3
 Fig.3 The graphic illustration for C-EVs promoting chondrogenic differentiation of HUCMSCs.2,3
Fig.3 The graphic illustration for C-EVs promoting chondrogenic differentiation of HUCMSCs.2,3









