STRA6 encodes a 75 kDa multi-transmembrane protein (receptor for retinol uptake STRA6) expressed in embryonic and adult tissues with no sequence similarity to any known transporter, channel or receptor. The epithelium that constitutes the blood-tissue barrier expresses a particularly high level of STRA6, including the retinal pigment epithelium (RPE) of the eyes, the ependymal cells of the choroid plexus (CP) in the brain, and Sertoli cells of the testis. Biochemical studies have shown that STRA6 is a long-established cellular receptor for the serum retinol-binding protein 4 (RBP4). The systematic structural analysis determines that STRA6 has 9 transmembrane domains, 5 extracellular domains, and 4 intracellular domains. The domain necessary for binding to RBP is located between transmembrane domains VI and VII. It is shown that STRA6 catalyzes the release of retinol from RBP and promotes the translocation of retinol on the membrane and binding to CRBP1.
| Basic Information of STRA6 | |
| Protein Name | Receptor for retinol uptake STRA6 |
| Gene Name | STRA6 |
| Aliases | Retinol-binding protein receptor STRA6, Stimulated by retinoic acid gene 6 protein homolog |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q9BX79 |
| Transmembrane Times | 9 |
| Length (aa) | 667 |
| Sequence | MSSQPAGNQTSPGATEDYSYGSWYIDEPQGGEELQPEGEVPSCHTSIPPGLYHACLASLSILVLLLLAMLVRRRQLWPDCVRGRPGLPSPVDFLAGDRPRAVPAAVFMVLLSSLCLLLPDEDALPFLTLASAPSQDGKTEAPRGAWKILGLFYYAALYYPLAACATAGHTAAHLLGSTLSWAHLGVQVWQRAECPQVPKIYKYYSLLASLPLLLGLGFLSLWYPVQLVRSFSRRTGAGSKGLQSSYSEEYLRNLLCRKKLGSSYHTSKHGFLSWARVCLRHCIYTPQPGFHLPLKLVLSATLTGTAIYQVALLLLVGVVPTIQKVRAGVTTDVSYLLAGFGIVLSEDKQEVVELVKHHLWALEVCYISALVLSCLLTFLVLMRSLVTHRTNLRALHRGAALDLSPLHRSPHPSRQAIFCWMSFSAYQTAFICLGLLVQQIIFFLGTTALAFLVLMPVLHGRNLLLFRSLESSWPFWLTLALAVILQNMAAHWVFLETHDGHPQLTNRRVLYAATFLLFPLNVLVGAMVATWRVLLSALYNAIHLGQMDLSLLPPRAATLDPGYYTYRNFLKIEVSQSHPAMTAFCSLLLQAQSLLPRTMAAPQDSLRPGEEDEGMQLLQTKDSMAKGARPGASRGRARWGLAYTLLHNPTLQVFRKTALLGANGAQP |
STRA6 is particularly abundantly expressed in the eyes, where it is primarily localized in the basolateral membrane of the retinal pigment epithelium, as well as in the female reproductive organs and placenta. Mutations in the human STRA6 gene are associated with Matthew-Wood Syndrome (MWS), which presents ocular defects ranging from mild microphthalmia to anophthalmia, as well as a range of other developmental abnormalities, including cardiac and pulmonary defects and cognitive deficits. STRA6 acts as a retinoid channel that promotes bidirectional flux of vitamin A between the extracellular and intracellular compartments. During this process, cellular accumulation of vitamin A is driven by the esterification of lecithin: retinol acyltransferase (LRAT). STRA6 missense mutations interfere with intracellular STRA6 protein transport or significantly reduce vitamin A uptake. However, STRA6 knockout mice only show ocular vitamin A deficiency but appeared healthy. The phenotype of these mice is similar to mice lacking the STRA6 ligand RBP4; RBP4 knockout mice show intraocular retinol deficiency and visual impairment.
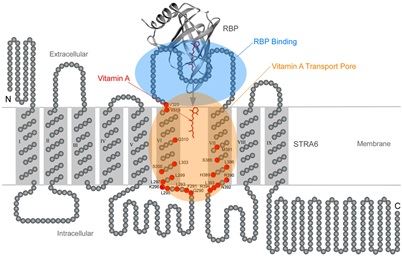 Fig.1 Schematic representation of the location of key positions in STRA6, whose acute modification blocks the transport of vitamin A by STRA6. The transmembrane topology model of STRA6 is described along with the crystal structure of holo-RBP. The key RBP binding domain is shaded in blue. Transmembrane domains and adjacent regions known as part of the vitamin A transport pore are shaded in orange. Residues whose acute modification hinders vitamin A transport by STRA6 are represented as red circles. (Kawaguchi, 2015)
Fig.1 Schematic representation of the location of key positions in STRA6, whose acute modification blocks the transport of vitamin A by STRA6. The transmembrane topology model of STRA6 is described along with the crystal structure of holo-RBP. The key RBP binding domain is shaded in blue. Transmembrane domains and adjacent regions known as part of the vitamin A transport pore are shaded in orange. Residues whose acute modification hinders vitamin A transport by STRA6 are represented as red circles. (Kawaguchi, 2015)
This article points out that retinol transport and cell signaling in STRA6 are largely interdependent and that both are associated with intracellular vitamin A metabolism.
This article suggests that zebrafish STRA6 has an intramembrane and nine transmembrane helices in a complex dimeric assembly, and calmodulin binds tightly to STRA6 in a non-canonical arrangement.
This review summarizes the basic molecular mechanisms mediated by plasma retinol binding protein (RBP) receptors and the various catalytic activities of this receptor and compares this transport system with retinoid transport independent of RBP/STRA6.
This article reveals that STRA6-mediated vitamin A uptake is a necessary regulatory process for vitamin A uptake, suggesting that it may be a true vitamin A transporter, which has important implications for diseases associated with impaired blood vitamin A homeostasis.
This article draws three conclusions: (1) the loss of STRA6 in T cells does not affect the immune response of T cells; (2) the uptake of STRA6-independent vitamin A compensates for the lack of STRA6 in lymphoid organs under vitamin A sufficient conditions in mice; (3) Even in the case of adequate vitamin A, STRA6 is essential for the uptake of vitamin A in the eye.
In the past few years, membrane protein studies have advanced significantly. Based on our versatile Magic™ membrane protein production platform, we could offer a series of membrane protein preparation services for worldwide customers in reconstitution forms as well as multiple active formats. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-STRA6 antibody development services.
Creative Biolabs has successfully generated many functional membrane proteins for our global customers during the past years. We are happy to accelerate the development of our clients’ programs with our one-stop, custom-oriented service. Please feel free to contact us for more detailed information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.