Biomedical research demands advanced in vitro models to simulate biological processes precisely and speed up the creation of new therapeutic agents. Two-dimensional cell cultures serve as foundational tools yet fail to fully represent the complex physiological characteristics of tissues found in living organisms. The development of Multicellular Tumor Spheroids (MCTS) represents an advance in three-dimensional cell culture models that effectively links conventional 2D cultures with complex animal models in vivo. MTS represent three-dimensional clusters of cancer cells that replicate in vivo tumor characteristics better than traditional two-dimensional cultures do. Cancer cells undergo self-assembly to form spheroids through interactions between themselves and both cell-cell and cell-extracellular matrix (ECM) contacts.
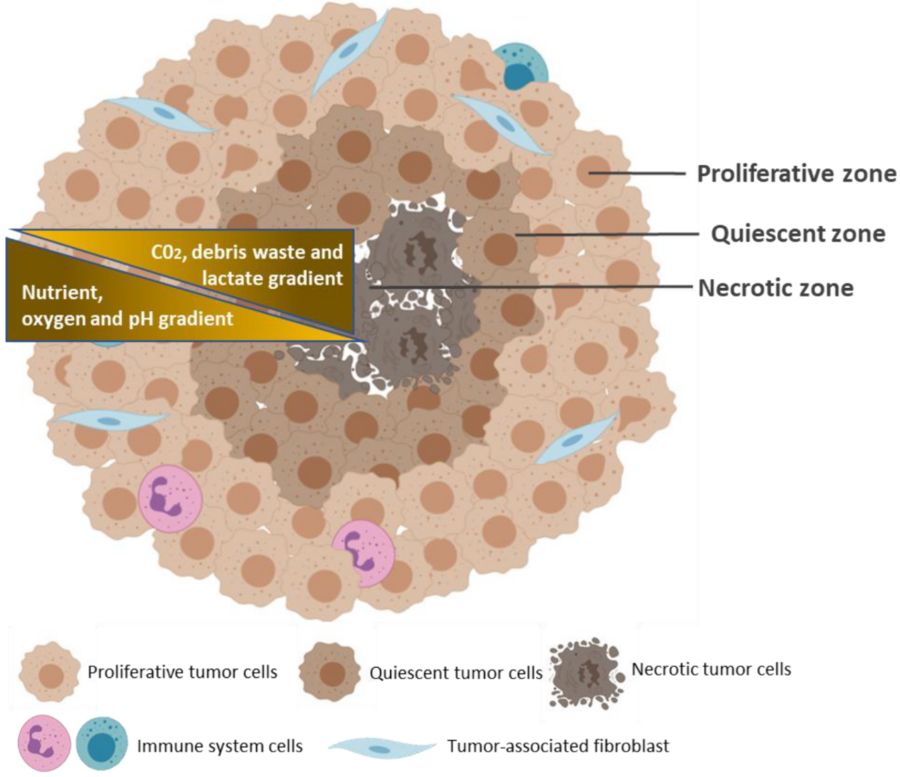 Figure 1 Typical structure of a multicellular tumor spheroid.1,3
Figure 1 Typical structure of a multicellular tumor spheroid.1,3
Multicellular Tumor Spheroid Types
MCTS are categorized by composition and scaffold use:
|
Spheroid Type
|
Description
|
Primary Applications
|
|
Homotypic Spheroids
|
Single cancer cell type aggregates.
|
Basic cancer biology, immunological disease drug screening, biomarker discovery.
|
|
Heterotypic Spheroids
|
Cancer cells co-cultured with other TME cells (e.g., fibroblasts, immune cells).
|
Studying tumor-stroma/immune interactions, angiogenesis, metastasis, and TME-targeted drugs.
|
|
Scaffold-Free Spheroids
|
Formed by cell self-assembly without external materials (e.g., hanging drop, ULA plates).
|
Common for drug development, basic spheroid biology.
|
|
Scaffold-Based Spheroids
|
Formed within or on biocompatible hydrogels/matrices (e.g., alginate, gelatin).
|
Studying cell-matrix interactions, simulating specific TMEs (e.g., hypoxic GBM regions), engineering complex tissues.
|
How to Calculate Volume of a Multicellular Tumor Spheroid?
The accurate measurement of MCTS volume remains essential to monitor growth kinetics and drug evaluation alongside standardizing experimental protocols. Researchers calculate spheroid volumes through bright-field, phase-contrast, or fluorescent imaging by measuring cross-sectional radii or diameters assuming perfect spherical shape. The method of estimating spheroid volumes becomes unreliable when dealing with large or irregularly shaped spheroids that evolve into more complex forms. To achieve precise volume calculation, advanced 3D imaging techniques are employed:
-
Confocal and Light Sheet Fluorescence Microscopy
Optical z-stacks generated by these techniques provide complete 3D information. Some software tool processes z-stacks to segment images and reconstruct spheroid volumes precisely without any predetermined geometric assumptions.
-
Optical Coherence Tomography (OCT)
Optical Coherence Tomography (OCT) provides high-resolution imaging with deep penetration capabilities to visualize the complete 3D structure of spheroids. This technology performs accurate voxel-based volumetric measurements of irregular-shaped spheroids and identifies important internal characteristics like necrotic regions which indicate tumor progression and drug response.
|
Cell Type
|
Description
|
Representative Lines
|
|
Cancer Cell Lines
|
Form spheroids that accurately reflect tumor biology, including drug resistance and proliferation patterns.
|
HeLa (cervical cancer), A549 (lung cancer), MCF-7 (breast cancer), HCT116 (colon cancer)
|
|
Primary Tumor Cells
|
Isolated from patient tissues, retaining physiological relevance better than immortalized lines.
|
Patient-derived tumor cells (e.g., ovarian, glioma primary cells)
|
Multicellular Tumor Spheroids in Ovarian
Ovarian cancer stands as one of the deadliest gynecological malignancies with advanced stage diagnosis and frequent development of resistance to treatment. Multicellular aggregates serve as critical elements in the spread of ovarian cancer through peritoneal dissemination. Ovarian MCTS provide essential in vitro models for researchers to study complex processes. Studies indicate that ovarian cancer spheroids create stable formations resembling the three-dimensional structure of metastatic clusters and show resistance to paclitaxel treatment similar to what is observed in living organisms. Research on cisplatin-resistant SKOV3 ovarian epithelial cancer spheroids demonstrates unique biophysical properties when compared to parental SKOV3 spheroids. The mechanical properties of CR spheroids change as indicated by their larger area and perimeter, decreased sphericity and modified Young's modulus. The research demonstrates the ability of MCTS to deliver essential biophysical information about how aggregates form and metastasize along with chemoresistance mechanisms in ovarian cancer.
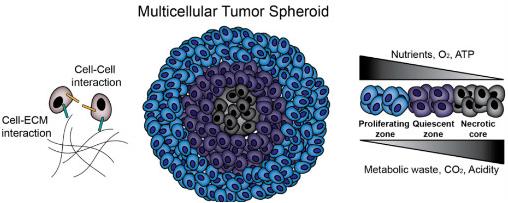 Figure 2 Multicellular tumor spheroids (MCTS) biology.2,3
Figure 2 Multicellular tumor spheroids (MCTS) biology.2,3
What is Alginate Gelatin Multicellular Tumor Spheroids?
MCTS can be scaffold-free but using biocompatible hydrogels creates a microenvironment that is both controllable and adjustable to resemble native tumor stroma. Alginate-gelatin hydrogels stand out as a widely used effective solution for developing scaffold-based MCTS.
-
Alginate: Alginate functions as a naturally occurring polysaccharide that maintains bioinertness while achieving superior mechanical stability through interaction with divalent ions like calcium. It creates a durable structural framework which encapsulates cells and permits exchange of nutrients and gases.
-
Gelatin: Gelatin remains a denatured collagen version which preserves essential cell attachment sites like RGD sequences critical for cellular attachment and signal transmission. Gelatin helps model the extracellular matrix while enhancing cellular survival and spheroid development.
Researchers have developed a composite hydrogel from alginate and gelatin that features customizable mechanical properties and biochemical signals which replicate the tumor stroma's microscopic structure. The engineered 3D environment sustains cancer cells in the formation of stable and viable MCTS for extended durations that exceed 30 days.
Differences Between Multicellular Tumor Spheroids and Other 3D Models
|
Feature/Model
|
2D Cell Culture (Monolayer)
|
Multicellular Tumor Spheroids (MCTS)
|
Organoids
|
Patient-Derived Xenografts (PDX)
|
|
Complexity/Architecture
|
Simplistic, flat monolayer
|
3D spherical aggregates, cell-cell interactions, gradients
|
3D, self-differentiating, resembles organ structure/function
|
Full in vivo tumor microenvironment, vascularized
|
|
In Vivo Mimicry
|
Low (lacks physiological context)
|
Moderate (mimics avascular tumor nodules, gradients, cell interactions)
|
High (mimics tissue architecture, cell types, some organ function)
|
Very High (retains original tumor heterogeneity, TME, vascularity)
|
|
Ease of Production
|
High (simple, fast)
|
Moderate (various methods: hanging drop, ULA plates, bioprinting)
|
Moderate to High (complex protocols, specific growth factors)
|
Low (requires animal models, ethical considerations, high cost)
|
|
Cost
|
Low
|
Moderate
|
High
|
Very High
|
|
Throughput
|
High (amenable to automation)
|
Moderate to High (with automation/microfluidics)
|
Low to Moderate
|
Low
|
|
Cell Source
|
Established cell lines, primary cells
|
Established cell lines, primary cells, patient-derived cells
|
Stem cells (ESCs, iPSCs), primary tissues/biopsies
|
Patient tumor tissue implanted into immunodeficient mice
|
|
Key Applications
|
Basic cell biology, high-throughput screening
|
Drug screening, drug penetration, TME studies, radiobiology
|
Disease modeling, drug discovery, regenerative medicine
|
Preclinical drug, personalized medicine, biomarker discovery
|
|
Limitations
|
Lacks 3D context, poor drug predictability
|
Lacks vasculature, limited TME complexity
|
Lacks full systemic interactions, TME often simplified
|
High cost, low throughput, ethical concerns, human relevance often debated
|
Multicellular Tumor Spheroids: An Underestimated Tool
MCTS have become essential tools across cancer research and drug development because they offer high physiological relevance.
-
Improved Efficacy Testing: MCTS offer an improved evaluation system for anticancer drug efficiency because they replicate in vivo drug penetration difficulties and resistance processes. The utilization of MCTS results in more accurate clinical outcome predictions and decreased drug candidate attrition rates.
-
Toxicity Profiling: Researchers use them for in vitro toxicity evaluations to study harmful effects on 3D tissue-like constructs.
Cancer Biology Research
-
Studying Tumor Microenvironment (TME): MCTS co-culture with various stromal cells including fibroblasts, endothelial cells, and immune cells enables researchers to model complex TME interactions which are essential for studying tumor growth, invasion, and metastasis.
-
Investigating Drug Resistance Mechanisms: The natural gradients found within MCTS create adaptive cellular responses that serve as an effective model for studying resistance mechanisms related to hypoxia and nutrient deprivation along with cell-cell interactions during chemo- and radiotherapy.
Frequently Asked Questions
Q: What are the primary advantages of MCTS over traditional 2D cell cultures?
A: MCTS offer several advantages including the creation of physiological oxygen and nutrient gradients that match in vivo environments and support complex cellular interactions while providing precise gene expression data and improved simulation of drug penetration and resistance mechanisms observed in living organisms. This leads to more predictive preclinical data.
Q: How are MCTS typically formed?
A: To create MCTS researchers apply several methods which include the hanging drop technique, use of ultra-low attachment plates, spinner flask cultures and they also implement magnetic and acoustic levitation along with state-of-the-art procedures like 3D bioprinting and microfluidic systems. The choice of spheroid creation technique depends on the specific features needed in the spheroids and the amount of experimental throughput required.
Q: What are the challenges associated with using MCTS in research?
A: Research using MCTS faces several challenges such as producing spheroids with consistent dimensions and shapes as well as preserving cell viability over extended periods for specific cell types while also contending with the absence of natural blood vessel networks which perfusion systems aim to mitigate alongside the difficulty of analyzing three-dimensional culture data compared to simpler two-dimensional cultures.
Conclusion
Multicellular Tumor Spheroids consist of cancer cell aggregates forming 3D structures which accurately replicate the architecture and physiological behavior of tumors in living organisms. MCTS establish essential oxygen and nutrient gradients while conventional 2D cultures do not and enable genuine cell-cell interactions leading to varying cell proliferation rates and development of drug resistance areas. MCTS' increased biological relevance serves as an advanced model for preclinical drug discovery by offering precise predictions regarding drug effectiveness and toxicity while monitoring penetration levels. The study of cancer biology benefits greatly from their use in analyzing tumor microenvironment interactions while exploring metastasis patterns and drug resistance pathways. Current research efforts concentrate on sophisticated generation methods and the combination of biosensors with complex co-culture systems which establish MCTS as fundamental for creating advanced cancer treatment solutions.
Overview of What Creative Biolabs Can Provide
Creative Biolabs stands at the forefront of biotechnology by delivering complete innovative solutions for cancer research and drug discovery through advanced 3D biology models like Multicellular Tumor Spheroids. We utilize our extensive expertise and advanced platforms to deliver:
Custom Services
Culture Products
Creative Biolabs is committed to helping our clients accelerate their preclinical drug discovery initiatives and gain deeper insights into cancer biology through our superior 3D modeling capabilities.
References
-
Pinto B, Henriques A C, Silva P M A, et al. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics, 2020, 12(12): 1186. https://doi.org/10.3390/pharmaceutics12121186
-
Kamatar A, Gunay G, Acar H. Natural and synthetic biomaterials for engineering multicellular tumor spheroids[J]. Polymers, 2020, 12(11): 2506. https://doi.org/10.3390/polym12112506
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Typical structure of a multicellular tumor spheroid.1,3
Figure 1 Typical structure of a multicellular tumor spheroid.1,3
 Figure 2 Multicellular tumor spheroids (MCTS) biology.2,3
Figure 2 Multicellular tumor spheroids (MCTS) biology.2,3