CRISPR-Cas9 technology has been used in many broad-based applications such as therapeutics, industry, and basic research. Creative Biolabs provides B cell a comprehensive service to establish gene-modified B cells with specific genomic editing events at a safe harbor locus. Our dedicated team has developed a CRISPR system that is designed to integrate a gene of interest or any other genetic element into the AAVS1 safe harbor site in human chromosome 19 via homologous recombination. Insertion of an expression cassette into the AAVS1 safe-harbor site can be used to introduce a transgene for overexpression and rescue studies without disrupting the expression of endogenous genes. These modified B cells can produce specific therapeutic proteins needed for a specific disease through precision genomic editing.
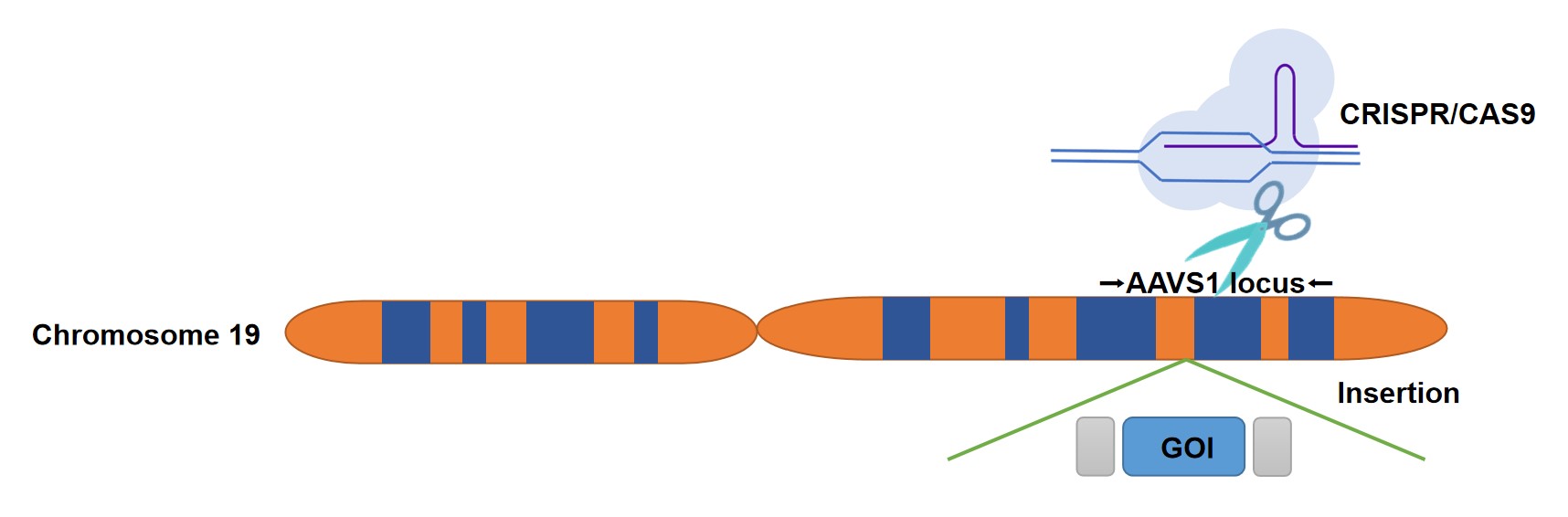 Fig.1 Genomic editing at the AAVS1 locus by CRISPR/Cas9. (Creative Biolabs)
Fig.1 Genomic editing at the AAVS1 locus by CRISPR/Cas9. (Creative Biolabs)
Creative Biolabs provides researchers with a one-stop service for engineering B cell—from experimental design to cell line development and validation. We offer tools and solutions for every step in the B cell engineering workflow. With decades of experience in cell engineering, our in-house team delivers unique solutions to support even the most demanding projects. Save yourself time and hassle and have Creative Biolabs handle all aspects of your B cell engineering workflow, from start to finish. The workflow of B cell engineering is as follows.
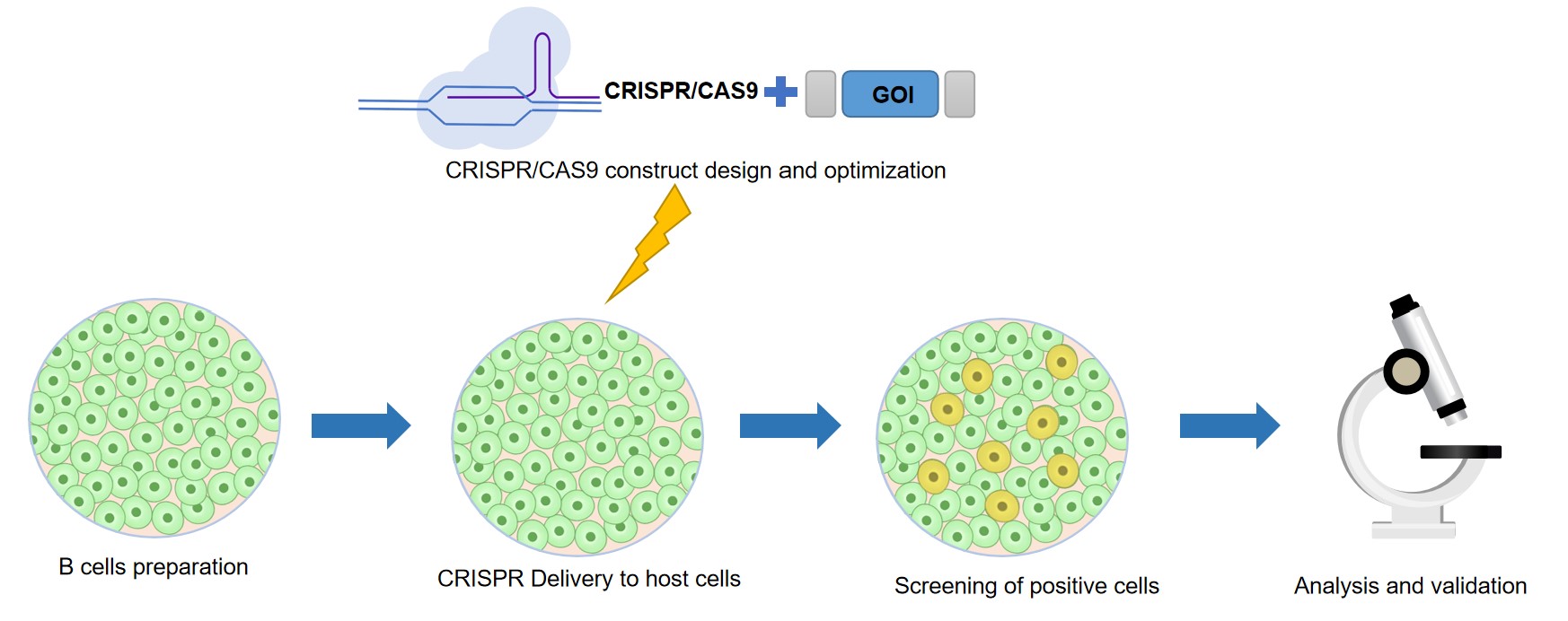 Fig.2 Experimental workflow of cell engineering by CRISPR/Cas9. (Creative Biolabs)
Fig.2 Experimental workflow of cell engineering by CRISPR/Cas9. (Creative Biolabs)
In addition to AAVS1 safe harbor site, we also provide other selections of safe harbor sites including
Creative Biolabs offers several levels of affordable custom services for designing, creating, and validating engineered B cells by a CRISPR/Cas9-based genome editing tool. Partner with us to get reliable gene-edited B cells:
Any requirements please contact us.
Data: CRISPR/Cas9 editing of primary human B cells at AAVS1 locus.
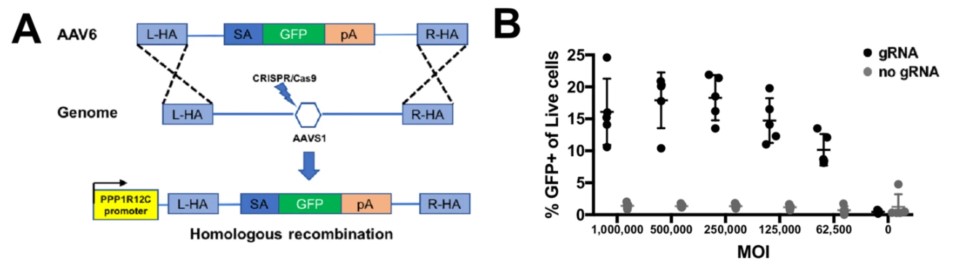 Fig.3 A schematic strategy and the efficiency of CRISPR/CAS9 editing in primary human B cells. (Johnson, et al., 2018)
Fig.3 A schematic strategy and the efficiency of CRISPR/CAS9 editing in primary human B cells. (Johnson, et al., 2018)