CNAG4 is encoded by the CNAG4 gene, and is also known as Cyclic nucleotide-gated cation channel alpha-4, Cyclic nucleotide-gated channel alpha-4, CNG channel alpha-4 and CNG4. It belongs to the Cyclic nucleotide-regulated channels family, which is ion channel whose activation is mediated by the binding of cGMP or cAMP to the channel protein. Meanwhile, structure analysis reveals that CNAG4 possesses a transmembrane channel core consisting of six α-helical segments (S1–S6), a reentrant pore (P) loop between S5 and S6, and cytosolic N- and C-termini.
| Basic Information of CNGA4 | |
| Protein Name | Cyclic nucleotide-gated cation channel alpha-4 |
| Gene Name | CNGA4 |
| Aliases | Cyclic nucleotide-gated channel alpha-4, CNG channel alpha-4, CNG4 |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q8IV77 |
| Transmembrane Times | 6 |
| Length (aa) | 575 |
| Sequence | MSQDTKVKTTESSPPAPSKARKLLPVLDPSGDYYYWWLNTMVFPVMYNLIILVCRACFPDLQHGYLVAWLVLDYTSDLLYLLDMVVRFHTGFLEQGILVVDKGRISSRYVRTWSFFLDLASLMPTDVVYVRLGPHTPTLRLNRFLRAPRLFEAFDRTETRTAYPNAFRIAKLMLYIFVVIHWNSCLYFALSRYLGFGRDAWVYPDPAQPGFERLRRQYLYSFYFSTLILTTVGDTPPPAREEEYLFMVGDFLLAVMGFATIMGSMSSVIYNMNTADAAFYPDHALVKKYMKLQHVNRKLERRVIDWYQHLQINKKMTNEVAILQHLPERLRAEVAVSVHLSTLSRVQIFQNCEASLLEELVLKLQPQTYSPGEYVCRKGDIGQEMYIIREGQLAVVADDGITQYAVLGAGLYFGEISIINIKGNMSGNRRTANIKSLGYSDLFCLSKEDLREVLSEYPQAQTIMEEKGREILLKMNKLDVNAEAAEIALQEATESRLRGLDQQLDDLQTKFARLLAELESSALKIAYRIERLEWQTREWPMPEDLAEADDEGEPEEGTSKDEEGRASQEGPPGPE |
CNAG4 belongs to ion channels and plays an essential role in the signal transduction of the olfactory and visual system. CNAG4 is activated when the concentration of the intracellular second messenger adenosine 3’,5’-monophosphate (cAMP) or guanosine 3’,5’-monophosphate (cGMP) is increased to the micromolar range, a process that is triggered by the specific signal transduction cascades within the receptor cells. In olfactory CNG channels, various modulatory effects of the CNGA4 and CNGB1b subunits have been identified by heterologous expression experiments. Both subunits make olfactory channels more sensitive to cAMP; thus, the concentration-activation relationship for cAMP is significantly shifted to lower concentrations. CNGA2 subunits promote the incorporation of CNGA4 and CNGB1b subunits into the plasma membrane and the CNBDs of CNGA4 and CNGB1b impart an increased apparent affinity for cAMP in heteromultimeric channels. In heterotetrameric channels, CNGA4 alters channel activity in response to cGMP and exhibit differential channel regulation in response to cGMP.
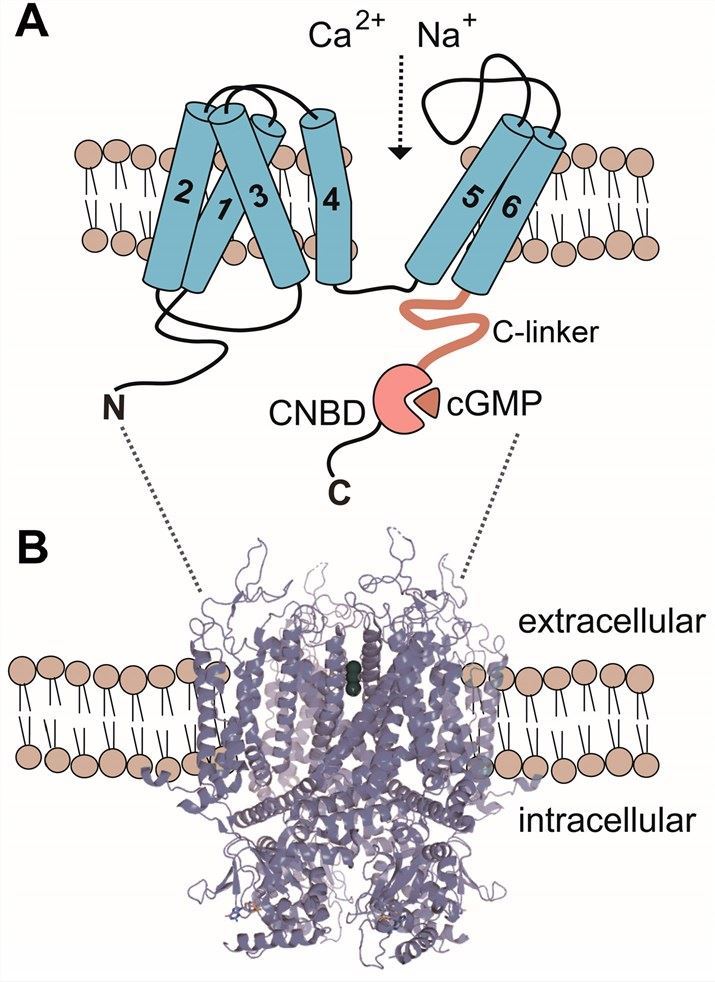 Fig.1 Membrane topology of CNGA4 channel subunits. (Michalakis, 2018)
Fig.1 Membrane topology of CNGA4 channel subunits. (Michalakis, 2018)
This article suggests that in heterotetrameric olfactory channels, cGMP binding to the CNGA2 subunits and the CNGA4 subunit, but not to the CNGB1b subunit, is sufficient to fully activate the channel.
This article reveals that Ca²⁺-dependent desensitization of the odor response in the OSNs mediated by the CNGA4 subunit is essential for normal odor sensation and adaptation of freely behaving mice, preventing saturation of the olfactory signal transduction machinery and extending the range of odor detection and discrimination.
This article shows that CNGA4 subunit accelerates the Ca²⁺-mediated negative feedback in olfactory signaling and allows rapid adaptation in this sensory system because Channels in excised membrane patches from the CNGA4 null mouse exhibited slower Ca²⁺-calmodulin-mediated channel desensitization.
This article provides an overview of the molecular properties of CNG channels and describes their physiological role in the phototransduction pathways. The authors also discuss insights into the pathophysiological role of CNG channel proteins that have emerged from the analysis of CNG channel-deficient animal models and human CNG channelopathies.
This article reports that in functional heterotetrameric channels not only the CNGA2 subunits and the CNGA4 subunit but also the CNGB1b subunit binds cyclic nucleotides and, moreover, also alone translates this signal to open the pore. In addition, this article shows that the CNGB1b subunit is the most sensitive subunit in a heterotetrameric channel to cyclic nucleotides and that it accelerates deactivation to a similar extent as does the CNGA4 subunit.
To obtain the soluble and functional target protein, the versatile Magic™ membrane protein production platform in Creative Biolabs enables many flexible options, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-CNGA2 antibody development services.
As a leading custom service provider in the field of membrane protein, within the last 10 years, Creative Biolabs has provided high-quality service to numerous customers for successfully accomplishing numerous challenging projects including generation of many functional membrane proteins. Please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.