Introduction of DPP6
DPP6 is a type II transmembrane protein that consists of a single transmembrane domain and a short intracellular N-terminus. DPP6 belongs to the prolyl oligopeptidase family of serine proteases. DPP6 is encoded by the DPP6 gene which is located at 7q36.2, and mutations of the gene can cause familial paroxysmal ventricular fibrillation 2 as well as mental retardation, autosomal dominant 33 (MRD33). The molecular mass is about 97 KDa.
| Basic Information of DPP6 | |
| Protein Name | Dipeptidyl aminopeptidase-like protein 6 |
| Gene Name | DPP6 |
| Aliases | DPPX, Dipeptidyl aminopeptidase-related protein, Dipeptidyl peptidase 6, Dipeptidyl peptidase IV-like protein, Dipeptidyl peptidase VI, DPP VI |
| Organism | Homo sapiens (Human) |
| UniProt ID | P42658 |
| Transmembrane Times | Single-pass membrane |
| Length (aa) | 865 |
| Sequence | MASLYQRFTGKINTSRSFPAPPEASHLLGGQGPEEDGGAGAKPLGPRAQAAAPRERGGGGGGAGGRPRFQYQARSDGDEEDELVGSNPPQRNWKGIAIALLVILVICSLIVTSVILLTPAEDNSLSQKKKVTVEDLFSEDFKIHDPEAKWISDTEFIYREQKGTVRLWNVETNTSTVLIEGKKIESLRAIRYEISPDREYALFSYNVEPIYQHSYTGYYVLSKIPHGDPQSLDPPEVSNAKLQYAGWGPKGQQLIFIFENNIYYCAHVGKQAIRVVSTGKEGVIYNGLSDWLYEEEILKTHIAHWWSPDGTRLAYAAINDSRVPIMELPTYTGSIYPTVKPYHYPKAGSENPSISLHVIGLNGPTHDLEMMPPDDPRMREYYITMVKWATSTKVAVTWLNRAQNVSILTLCDATTGVCTKKHEDESEAWLHRQNEEPVFSKDGRKFFFIRAIPQGGRGKFYHITVSSSQPNSSNDNIQSITSGDWDVTKILAYDEKGNKIYFLSTEDLPRRRQLYSANTVGNFNRQCLSCDLVENCTYFSASFSHSMDFFLLKCEGPGVPMVTVHNTTDKKKMFDLETNEHVKKAINDRQMPKVEYRDIEIDDYNLPMQILKPATFTDTTHYPLLLVVDGTPGSQSVAEKFEVSWETVMVSSHGAVVVKCDGRGSGFQGTKLLHEVRRRLGLLEEKDQMEAVRTMLKEQYIDRTRVAVFGKDYGGYLSTYILPAKGENQGQTFTCGSALSPITDFKLYASAFSERYLGLHGLDNRAYEMTKVAHRVSALEEQQFLIIHPTADEKIHFQHTAELITQLIRGKANYSLQIYPDESHYFTSSSLKQHLYRSIINFFVECFRIQDKLLTVTAKEDEEED |
Function of DPP6 Membrane Protein
DPP6 is an auxiliary subunit of the Kv4 family of voltage-gated K+ channels. It has been shown that DPP6 can enhance potassium channel surface expression and potently accelerate their kinetics. It is expressed predominantly in brain and exists as several splicing variants termed DPPX-L, DPPX-S, DPPX-N, and DPPX-O. DPP6 also participates in many biological processes, such as protein localization to plasma membrane and regulation of potassium ion transmembrane transport. On the other hand, DPP6 is also known for its large extracellular domain which is required for its export from the ER and expression and stabilization on the cell surface. At the same time, the short intracellular N-terminal of DPP6 and its transmembrane domains associates with and accelerates the recovery from inactivation of Kv4.2. In detail, a cysteine-rich motif included in extracellular domain plays an important role in protein folding of DPP6 that is required for transport of DPP6/Kv4.2 complexes out of the ER.
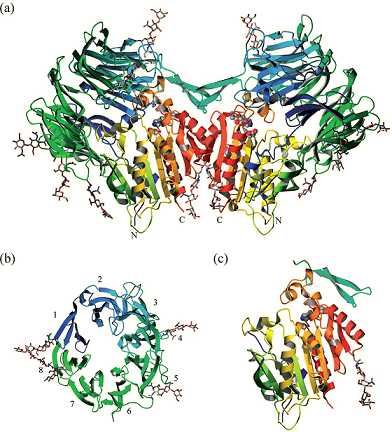 Fig.1 Structure of DPP6 membrane protein (Strop, 2004).
Fig.1 Structure of DPP6 membrane protein (Strop, 2004).
Application of DPP6 Membrane Protein in Literature
This article reports that KChIP2 isoforms and DPP6 proteins are not required for Kv4.3 activation by NS3623, but their presence influences the compound’s effects on the rate of current decay, dominating the overall effect as reflected by total charge transfer (AUC).
This article focuses on the behavioral consequences of DPP6 loss, the authors find that DPP6 knockout (DPP6-KO) mice are impaired in hippocampus-dependent learning and memory. Moreover, impaired synaptic development along with spatial learning and memory deficiencies in DPP6-KO mice.
Authors in this group studied 601 family members and probands: 286 DPP6 risk-haplotype positive (haplotype-positive) and 315 DPP6 risk-haplotype negative (haplotype-negative) individuals. However, they find there were no differences in electrocardiographic indices between haplotype-positives and haplotype-negatives, or between haplotype-positives with or without events.
Authors in this article identified a novel beta and alpha cell biomarker and developed a tracer for in vivo imaging of human insulin secreting cells. This provides a useful tool to non-invasively follow up intramuscularly implanted insulin secreting cells.
This article reveals that the extracellular domain and intracellular domain responsible for different biological function. In details, the extracellular domain is required for DPP6 export from the ER while intracellular domains impart the functional impact on Kv4.2.
DPP6 Preparation Options
To obtain the soluble and functional target protein, the versatile Magic™ membrane protein production platform in Creative Biolabs enables many flexible options, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-DPP6 antibody development services.
As a forward-looking research institute as well as a leading customer service provider in the field of membrane protein, Creative Biolabs has won good reputation among our worldwide customers for successfully accomplishing numerous challenging projects including generation of many functional membrane proteins. Please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.