Aptamers are being intensively investigated as specific targeting moieties in the biological area. Creative Biolabs provides a broad range of unique aptamer-based combination strategies to maximize the use of aptamers and current advanced technology to achieve the best diagnostic or therapeutic outcomes for academic research trials.
Aptamer-guided Active Targeting
The goal of surface modification of drug carriers by aptamers is to enhance internalization, specific drug accumulation, and retention in tumors via specific ligand-mediated interactions, therefore, increasing the therapeutic index. Aptamer-guided active targeting enables the increased delivery of therapeutic agents to tumors as well as a reduction in toxicity and side effects by minimizing the exposure of normal tissues to the therapeutic agent. As an active targeting ligand, aptamers are generally non-immunogenic or low-immunogenic. In brief, aptamer-mediated active targeting for cancer therapy can be categorized into four groups:
- Free aptamer (DNA/RNA) as molecularly targeted agents
- Aptamer-drug conjugates
- Aptamer-nanoparticle-drug delivery system
- Aptamer-mediated cancer nucleic acid therapy
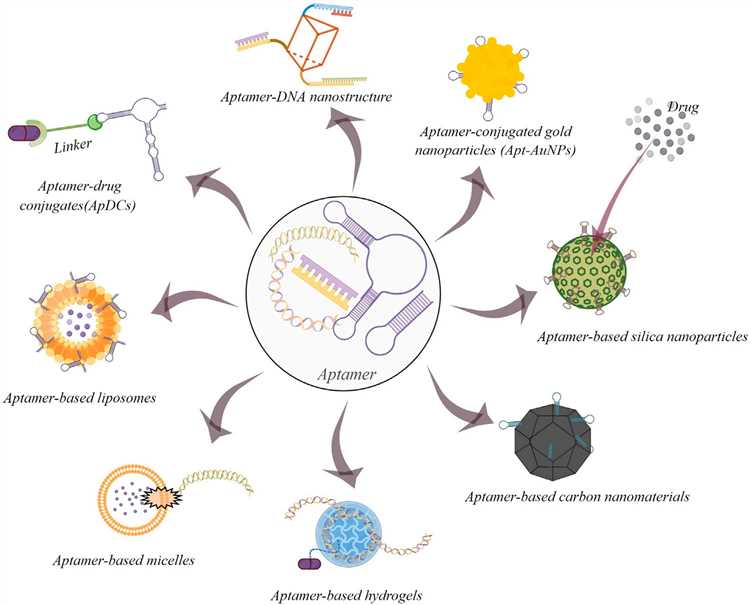 Fig.1 General scheme of drug delivery to tumor.1, 2
Fig.1 General scheme of drug delivery to tumor.1, 2
Our Aptamer-based Combination Strategies
Aptamers are short chemically synthesized oligonucleotides that can be further chemically modified to acquire desirable attributes for clinical applications. A wide range of diagnostic agents, therapeutic agents, and imaging agents can be used in combination with aptamers, via chemical or physical conjugation. For instance, aptamer-linked biosensors can be detected using various techniques such as fluorescence, chemiluminescence, electrochemistry, or immunoluminescence. This combination has been used for accurate and rapid detection of disease biomarkers, pathogens, antibiotics, toxins, pollutes, and other biomolecules or chemicals. Besides, non-invasive imaging of various disease markers using aptamer-modified probes has shown numerous advantages over traditional imaging techniques.
Advantages
- Provide synergistic or addictive effects beyond one single drug therapy
- Circumvent multiple drug resistance of cancer cells by targeted delivery of anticancer agents intracellularly
- Afford lower doses of individual drugs thus minimizing systematic side effects of individual agents
Combinational Solutions
- Aptamer-DNA combination
- Aptamer-miRNA combination
- Aptamer-shRNA combination
- Aptamer-siRNA chimera
Generating a chimera by linking an aptamer with siRNA, aptamer-guided siRNA delivery via receptor-mediated endocytosis serves as a promising approach for cancer-targeted RNAi therapy.
- Aptamer-drug combination
- Aptamer-biosensor combination
- Aptamer-boimaging combination
For detailed pricing information, please contact us for a custom quotation.
Published Data
1. A HER2-Targeting Aptamer-Drug Conjugate for Breast Cancer Therapy
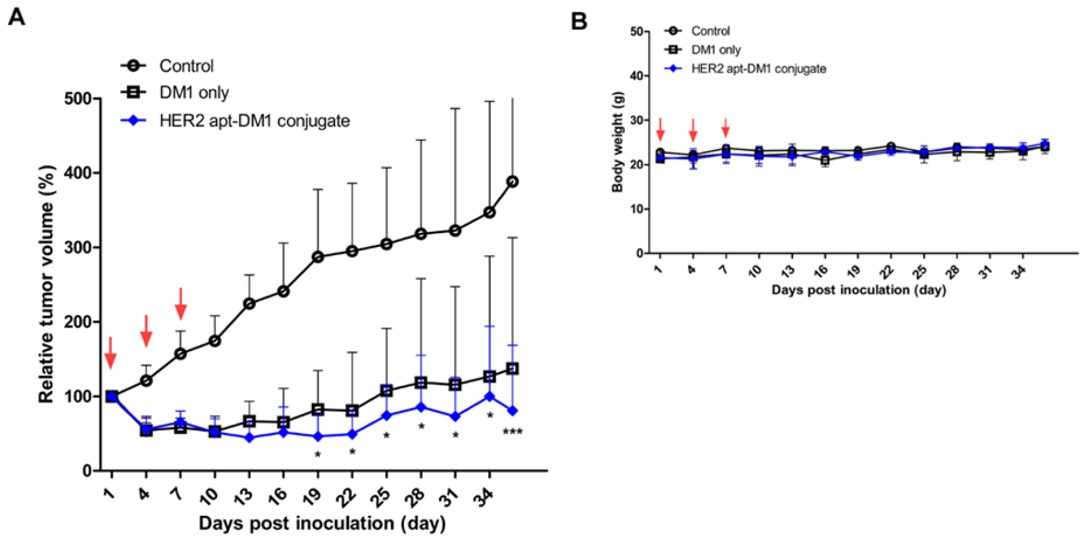 Fig.2 In vivo tumor growth inhibition by ApDC.3,2
Fig.2 In vivo tumor growth inhibition by ApDC.3,2
In this work, HER2-specific RNA aptamers were conjugated to anticancer drug mertansine (DM1), and the efficacy of the conjugate was assessed in breast cancer models with HER2 overexpression. The aptamer-drug conjugate (ApDC) was synthesized and characterized through mass spectrometry and HPLC. Cell-binding affinity and cytotoxicity were assessed through confocal microscopy and the WST-1 assay. In vivo anti-tumoral efficacy was tested in mice bearing BT-474 breast tumors with HER2 overexpression. The HER2-specific RNA aptamers selectively bound to HER2-positive BT-474 cells, but not HER2-negative MDA-MB-231 cells. The ApDC exhibited strong cytotoxicity against BT-474 cells, with minimal effects on MDA-MB-231 cells. In mouse xenograft models, the ApDC significantly inhibited tumor growth compared to controls, without causing notable body weight loss or hepatic toxicity. This research demonstrates the potential of the HER2 aptamer-DM1 conjugate as a targeted anticancer therapy and suggests its applicability in developing versatile, high-performance targeted anticancer drugs.
2. Bacteria Loaded with Aptamer-Drug Conjugates for Synergistic Therapy in Pancreatic Cancer
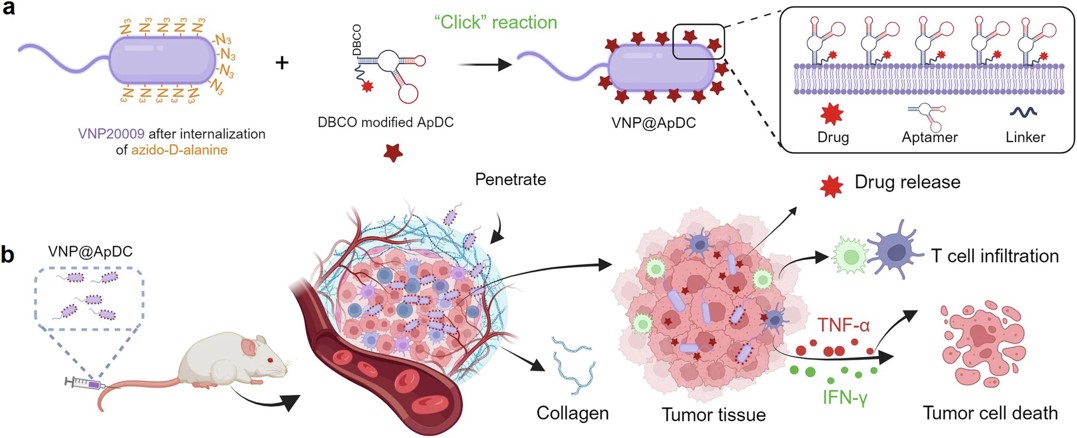 Fig.3 Schematic illustrating the construction of aptamer-drug conjugates-loaded bacteria and the mechanism against pancreatic tumors.4,2
Fig.3 Schematic illustrating the construction of aptamer-drug conjugates-loaded bacteria and the mechanism against pancreatic tumors.4,2
VNP20009, a genetically modified Salmonella typhimurium strain, has anaerobic preference but lacks specificity and is toxic. This study proposed a synergistic therapy combining VNP20009’s bacteria-mediated delivery with the targeted toxicity of ApDC. ApDC was conjugated to VNP20009 through one-step click chemistry, allowing the bacteria to target pancreatic cancer via anaerobic chemotaxis and aptamer-driven binding. The method enhanced serum stability of ApDC for up to 48 hours, increasing drug concentration at tumor sites compared to free drugs. The aptamer-mediated targeting tripled bacterial colonization at the tumor, resulting in greater tumor cell death and T cell infiltration. This strategy integrated chemotherapy and immunotherapy, significantly improving treatment effectiveness across animal models. Overall, it presents a promising synergistic approach for pancreatic cancer therapy by leveraging bacteria and ApDC.
References
- Gao, Fei, et al. "Recent advances in aptamer-based targeted drug delivery systems for cancer therapy." Frontiers in Bioengineering and Biotechnology 10 (2022): 972933.
- Distributed under Open Access license CC BY 4.0, without modification.
- Jeong, Hwa Yeon, et al. "Development of HER2-specific aptamer-drug conjugate for breast cancer therapy." International journal of molecular sciences 21.24 (2020): 9764.
- Xiao, Yu, et al. "Aptamer-drug conjugates-loaded bacteria for pancreatic cancer synergistic therapy." Signal Transduction and Targeted Therapy 9.1 (2024): 272.
For Research Use Only.