Aptamers can bind to the receptors on the cell membrane and mediate themselves or conjugated nanoparticles to enter into cells. Thus, aptamers can be served as ideal targeting ligands for drug delivery. With an extensive line of services to support your projects, Creative Biolabs is the best choice for high-quality, cost-effective collaborating / outsourcing options for your research programs. We can meet customer’s unmet needs with different kinds of our aptamer services. Our technology has a shorter development time compared to traditional methods of aptamer-targeted drug development and can be used to increase the specificity and improve the delivery of existing candidates resulting in the next generation of pharmaceuticals with improved pharmacodynamics and pharmacokinetics.
Aptamers for Targeted Drug Delivery
Aptamers are capable of binding to receptor molecules, which could lead to aptamer-based delivery systems and the usage of chemical tags alongside available chemical strategies to improve cellular uptake. Aptamer-mediated drug delivery has shown potential as a cancer treatment strategy. The primary considerations in the design of a targeted drug delivery system are to promote the accumulation of chemotherapeutic agents in the tumor tissue and overcome drug resistance. Ligands can guide nanocarriers to tumors and facilitate the transport of these nanocarriers into tumor cells, resulting in cell death. Depending on the compositions and preparation approaches, aptamer-targeted drug delivery systems can be divided into the following two main categories.
- Aptamer-small molecule conjugated systems-in which aptamers directly deliver drug molecules as both a carrier and a ligand
- Aptamer-nanomaterial conjugated systems-in which aptamers function together with nanoparticles (NPs) for targeted delivery of drugs
Advantage: Good chemical stability, isotropic properties
Disadvantage: Low drug loading, complex and costly procedures
Advantage: High loading capacity
Disadvantage: Unpredictable risks, complex and costly procedures
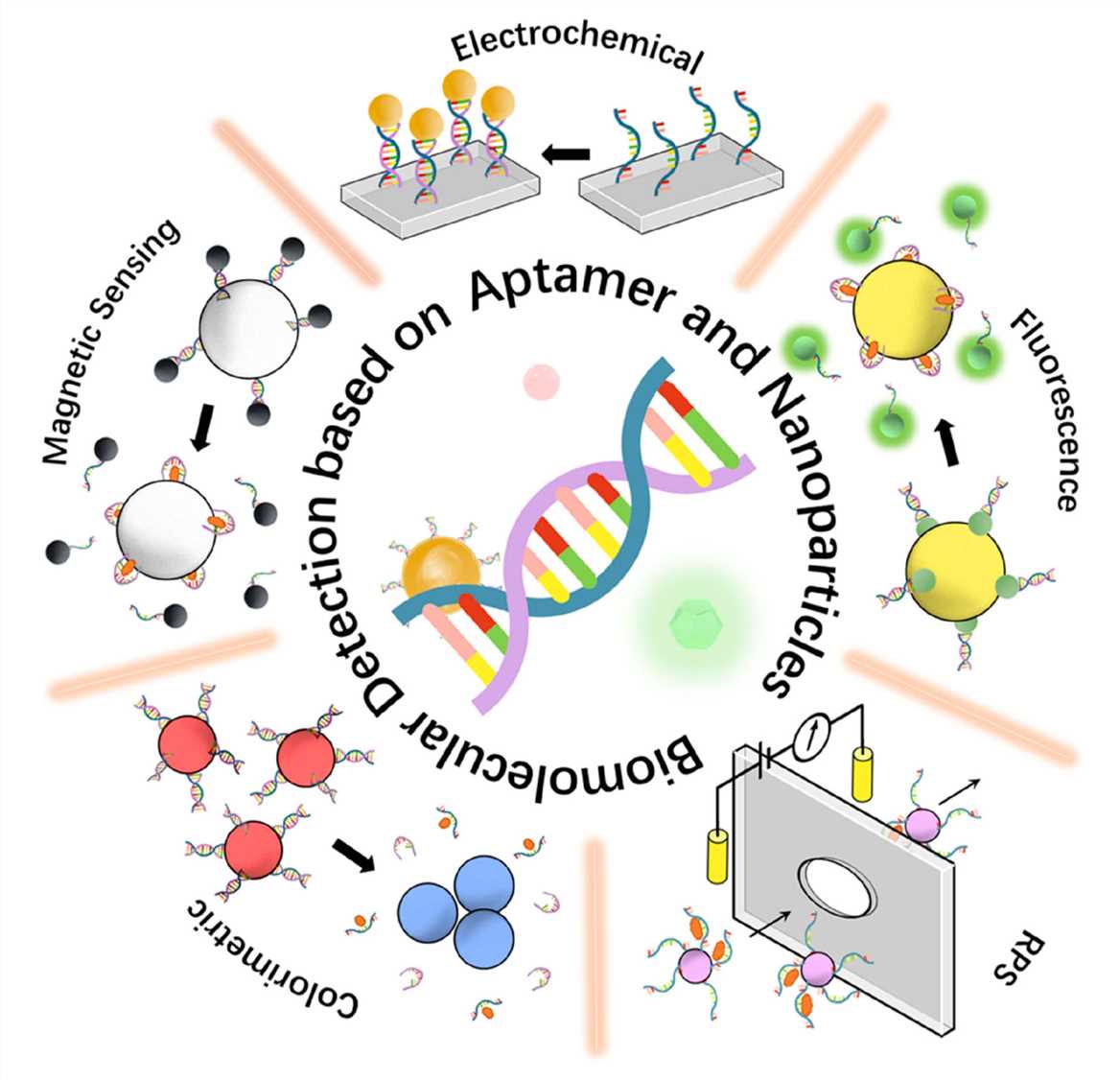 Fig.1 Different mechanisms of nanoparticle-aptamer-based biomolecular detection.1, 2
Fig.1 Different mechanisms of nanoparticle-aptamer-based biomolecular detection.1, 2
Our Strategies
The cell membrane is the barrier for the import and export of DNA/RNA to and from the cell. Cell membrane proteins from bacteria, viruses, and mammalian cells play an important role in pathogenesis and may be potential targets for the next generation of aptamers. With the basic developments in SELEX, different strategies have been utilized for generating cell-internalizing aptamers, such as whole-cell SELEX, protein SELEX, tissue-specific SELEX, and in vivo SELEX. Cell-SELEX is a straightforward way to generate internalizing aptamers because it involves direct interaction with whole cells and the selection of aptamer exclusively depends on the surface antigens. Besides, internalizing aptamers can also be developed via tissue penetration using the SELEX strategies. Oligonucleotide library is injected into the tail of the mouse. The targeted tissue will be dissected for the isolation of bound molecules.
Advantages for Aptamer Conjugation
- Aptamers can be selected to bind with a high degree of specificity to highly similar targets.
- Aptamer-facilitated binding can sometimes enable entry into cells or across the blood-brain barrier.
- Aptamers are non-immunogenic.
- Aptamers can be conjugated without affecting selective binding, enabling modifications to improve stability or labeling to assess aptamer-drug delivery.
Whether you have a cell type of interest or have identified a specific cell surface target, the first step in aptamer-mediated drug delivery is the discovery of selective aptamers. Creative Biolabs has discovered aptamers to cells, cell surface markers, small molecule drugs, and drug metabolites. Our advanced multiplex selection enables simultaneous discovery of selective aptamers to a panel of positive and negative targets, reducing the overall time and cost of aptamer discovery and improving aptamer selectivity. If you are interested in aptamer development for drug delivery, please feel free to contact us for more information.
Published Data
1. Aptamer-Functionalized Self-Assembled Nanomicelles for Efficient and Targeted Drug Delivery
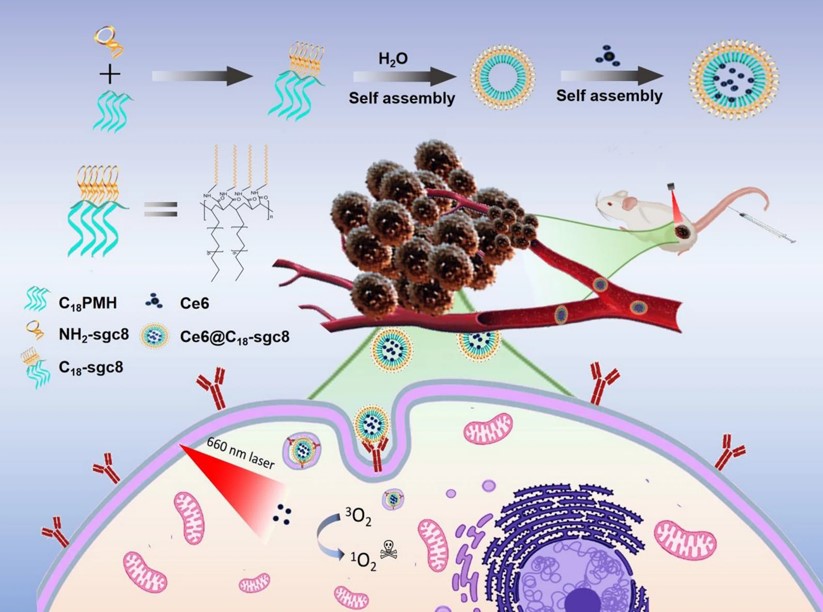 Fig.2 Schematic illustration of the synthesis method of C18-aptamer micelles and its application for targeted photodynamic therapy.3,2
Fig.2 Schematic illustration of the synthesis method of C18-aptamer micelles and its application for targeted photodynamic therapy.3,2
Nucleic acid aptamer-based nanomicelles hold significant promise for nanomedicine and nanotechnology, but their stability is a challenge, particularly when interacting with cell membranes in physiological environments, which can limit their cancer-targeting abilities. This study addressed that issue by developing a superstable micellar nanodelivery system. The system consists of an amphiphilic copolymer self-assembled micelle made from nucleic acid aptamers and the polyvalent hydrophobic poly(maleic anhydride-alt-1-octadecene) (C18PMH). When combined with a photosensitizer drug model, these C18-aptamer micelles effectively target and bind to tumor cells, enhancing the delivery of the photosensitizer for photodynamic therapy. They can also load other hydrophobic drugs and maintain strong therapeutic effects. This versatile C18-aptamer micelle platform offers a safer, more effective approach for cancer treatment by enabling efficient drug delivery.
2. Targeted Drug Delivery to Staphylococcus aureus Biofilms Using Aptamers
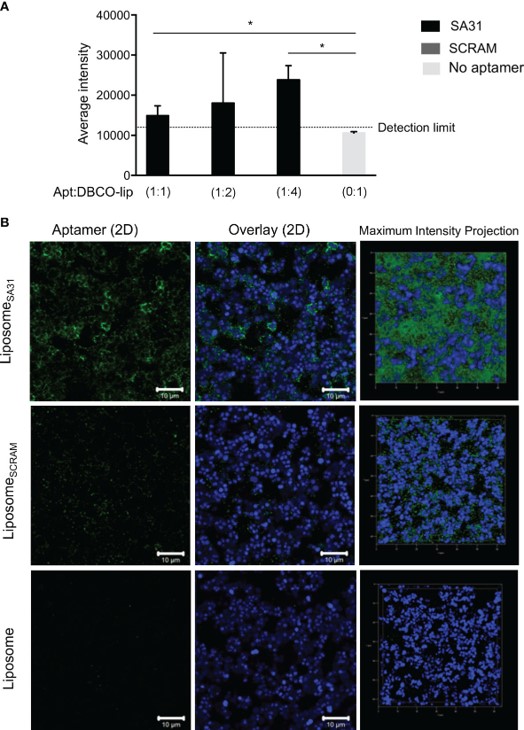 Fig.3 Accumulation of liposomes in S. aureus biofilm mediated by aptamer.4,2
Fig.3 Accumulation of liposomes in S. aureus biofilm mediated by aptamer.4,2
Treating Staphylococcus aureus biofilm infections with conventional antibiotics is difficult, as only sublethal doses can be safely administered, limiting their effectiveness. In this study, the researchers developed an aptamer-targeted liposomal drug delivery system for the localized delivery of antibiotics to S. aureus biofilms. They identified six DNA aptamers that specifically bound to S. aureus cells within biofilms, and one of these aptamers was shown to enhance liposome accumulation around S. aureus cells in the biofilm. The aptamer-targeted liposomes, encapsulating a combination of antibiotics commonly used to treat methicillin-resistant S. aureus (MRSA) infections, were demonstrated to successfully eradicate S. aureus biofilms after 24 hours of in vitro treatment. The findings suggest that aptamer-targeted drug delivery of antibiotics could be a promising new strategy for effectively treating S. aureus biofilm infections.
References
- Xu, Ruiting, et al. "Recent Advances in Biomolecular Detection Based on Aptamers and Nanoparticles." Biosensors 13.4 (2023): 474.
- Distributed under Open Access license CC BY 4.0, without modification.
- Chen, Ganghui, et al. "Aptamer-based self-assembled nanomicelle enables efficient and targeted drug delivery." Journal of Nanobiotechnology 21.1 (2023): 415.
- Ommen, Pernille, et al. "Aptamer-targeted drug delivery for Staphylococcus aureus biofilm." Frontiers in cellular and infection microbiology 12 (2022): 814340.
For Research Use Only.

