Creative Biolabs has been focusing on the development of high-quality in vitro diagnostic (IVD) antibodies for the diagnosis of a diversified battery of cancers. We offer contract antibody development services to help obtain highly specific antibodies that are developed against different biomarkers of pancreatic cancer. Particularly, ribonucleoside reductase subunit M1 (RRM1) is a promising biomarker for gemcitabine response and prognosis in pancreatic cancer patients.
Introduction of RRM1
Ribonucleotide reductase (RNR) is a multimeric enzyme catalyzing the conversion of ribonucleoside diphosphates to deoxyribonucleoside diphosphates in the de-novo metabolic pathway of endogenous nucleotides. RRM1, the large subunit of human ribonucleotide reductase, contains the substrate binding sites and binding sites for allosteric regulators. It is involved in the regulation of cell proliferation, cell migration, tumor and metastasis development, and the synthesis of deoxyribonucleotides for DNA synthesis. Moreover, it is also a cellular target for the chemotherapeutic agent, gemcitabine. RRM1 has been studied in a large number of patients with different types of cancer, such as non-small-cell lung cancer, pancreatic cancer, breast cancer, and biliary tract cancer, to establish its prognostic or predictive value when patients were treated with gemcitabine.
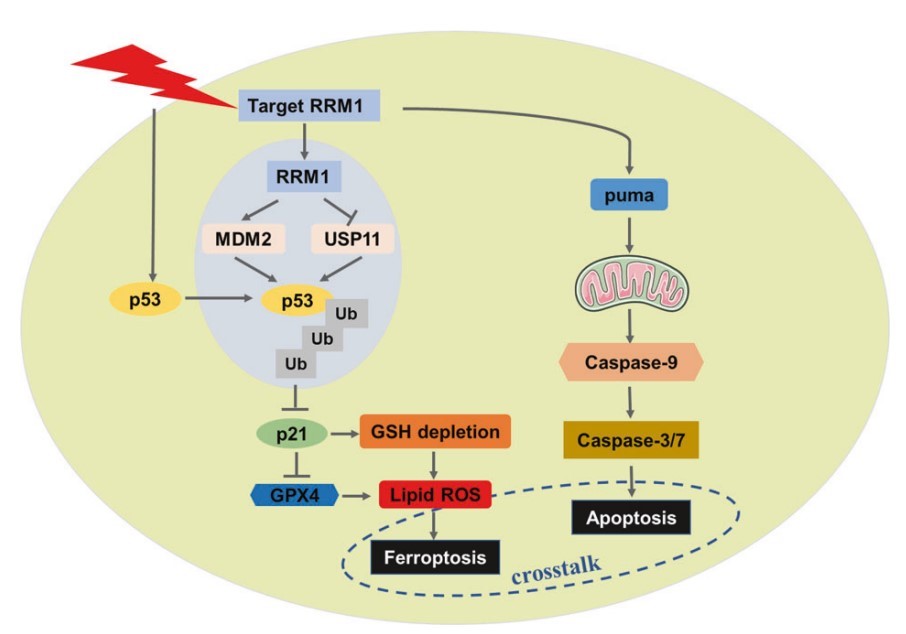 Fig.1 The mechanism of RRM1-mediated radiosensitization of cancer cells.1
Fig.1 The mechanism of RRM1-mediated radiosensitization of cancer cells.1
RRM1 Marker for Pancreatic Cancer
Pancreatic cancer remains a lethal disease with brief survival especially in patients with advanced disease. Standard treatment for unresectable pancreatic cancer is gemcitabine, although only a quarter of patients benefit from this chemotherapy. Therefore, predictive markers of gemcitabine resistance are important to allow effective treatment of these tumors and improved patient selection. Studies have suggested that RRM1 protein expression could be used to identify patients who would benefit from gemcitabine treatment. For instance, a clinical study reported that patients with advanced pancreatic carcinoma who exhibited high levels of RRM1 expression were found to have poor survival rates following gemcitabine treatment, whereas patients with low RRM1 expression treated with gemcitabine had a significantly better survival than did untreated patients.
IVD Antibody Development Services Targeting RRM1 Marker
As detection of RRM1 expression may be a promising biomarker for gemcitabine response and prognosis in pancreatic cancer patients, IVD antibodies against RRM1 marker can be developed to help the prognosis and diagnosis of pancreatic cancer, and to improve patient selection. At Creative Biolabs, we provide polyclonal, monoclonal, and recombinant antibody development services for diagnostic use. These antibodies can be customized for development of different immunoassay formats, including ELISAs, immunohistochemistry, western blot, and so on. With our versatile IVD platform, Creative Biolabs is proud to develop novel RRM1-specific (paired) antibodies from scratch to commercial IVD kit (we can also start with provided antibody candidates).
As a well-recognized expert in the field of IVD antibody development, Creative Biolabs has achieved a lot of projects for our clients based on our extensive experience and advanced platform. We are therefore confident in offering the best services to customers all over the world. Contact us to discuss your project and experience the great value of our services.
Reference
- Gao, Yang, et al. "Knockdown of RRM1 in tumor cells promotes radio-/chemotherapy induced ferroptosis by regulating p53 ubiquitination and p21-GPX4 signaling axis." Cell Death Discovery 8.1 (2022): 343. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.