The journey begins by assessing your project's scientific and commercial viability. This involves analyzing the target, defining clinical utility, and setting performance specifications. We review market and regulatory pathways to strategically position your IVD. This foundational step establishes objectives and identifies early challenges.
Creative Biolabs is a technology-driven service provider specializing in high-quality antibody development and in vitro diagnostic (IVD) immunoassay development for a global clientele. Our extensive expertise in antibody production allows us to deliver superior antibody ( or antibody pairs) and offer custom production/validation services against a broad spectrum of disease biomarkers.
For immunoassay development, our IVD immunoassay kit development expertise lies in different formats, including lateral flow, chemiluminescence assays, ELISA tests, turbidimetric assays, and rapid tests. Our highly customizable services ensure the development of robust and specific assays for diverse applications.
Introduction to IVD Immunoassay
IVD immunoassays are foundational to modern healthcare, enabling rapid and precise detection of specific biomarkers in biological samples. These powerful tools are indispensable for disease diagnosis, prognosis, treatment monitoring, and public health surveillance. Leveraging the highly specific binding between antibodies and antigens, immunoassays can identify and quantify substances present even in minute concentrations, from proteins and hormones to infectious agents and drugs.
Common IVD immunoassay formats include:
ELISA
A highly versatile and widely adopted immunoassay format, ELISA tests use an enzyme-linked detection system to quantify antigens or antibodies, adaptable for various applications in both research and clinical diagnostics.
Lateral Flow Assays (LFAs)
These are rapid, paper-based tests designed for qualitative or semi-quantitative detection, often used for point-of-care diagnostics due to their simplicity, speed, and portability.
Chemiluminescence Immunoassays (CLIAs)
Highly sensitive and quantitative, CLIAs utilize a chemiluminescent reaction to produce a light signal, making them ideal for laboratory-based detection of low-concentration analytes with broad dynamic ranges.
Turbidimetric Assays
These assays measure changes in turbidity or light scattering caused by antigen-antibody complex formation, offering a rapid and quantitative method, particularly useful in clinical chemistry for measuring proteins and other analytes.
Immunochromatographic Assays (ICAs)
Often synonymous with lateral flow tests, ICAs represent a broad category of rapid diagnostic tests that rely on the immunochromatographic principle for visual detection of analytes, crucial for rapid screening.
Rapid Tests
This general term encompasses various quick-turnaround diagnostic assays, including LFAs and some ELISA variants, designed for immediate results outside of a central laboratory setting, supporting timely clinical decisions.
IVD Immunoassay Development Services Provided by Creative Biolabs
Creative Biolabs provides a complete, end-to-end IVD immunoassay development service, meticulously designed to guide your diagnostic concept from initial idea to a validated, manufacturing-ready product. Our comprehensive approach ensures optimal performance and regulatory alignment, reflecting our commitment to quality and scientific excellence. We offer a modular service allowing for tailored support or complete program management.
- Target & Biomarker Assessment
- Reagent Sourcing & Development
- Assay Design & Optimization
- Analytical Validation
- Manufacturing & QC Support
- Regulatory Documentation Assistance
Our Immunoassay Development Phases
Creative Biolabs executes IVD immunoassay development through a structured, phase-gated, and regulated process. We offer comprehensive custom solutions or flexible support for specific project needs. Our team assists with assay design, raw material selection, and robust protocol establishment. Systematic optimization ensures peak performance. Throughout, design reviews conclude each phase, confirming milestones and maintaining your control.
Phase 1: Project Initiation and Feasibility Assessment
Phase 2: Reagent Discovery and Optimization
Developing core immunoassay components is paramount. This phase focuses on identifying, generating, and optimizing critical biological reagents: antibodies and antigens. We engineer high-affinity binders, optimizing conjugation to labels or supports. Careful buffer and blocking strategies ensure robust, consistent reagent performance.
Phase 3: Assay Design and Prototyping
The immunoassay's conceptual design takes tangible form. We select the optimal format (e.g., ELISA, lateral flow) based on your application. Initial prototypes are assembled and tested with controls. This iterative process quickly identifies issues, assesses preliminary sensitivity, and refines assay configuration for a functional proof-of-concept.
Phase 4: Assay Optimization and Validation
This pivotal stage rigorously defines and fine-tunes assay performance. Comprehensive experiments optimize critical parameters like reagent concentrations and incubation times. Analytical validation determines key characteristics: sensitivity, specificity, linearity, precision, and robustness. Interference studies ensure reliable results across diverse matrices.
Phase 5: Manufacturing Transfer and Quality Control
Successful IVD development culminates in a scalable, consistent manufacturing process. We prepare all necessary documentation, including detailed SOPs and batch records, crucial for seamless technology transfer. We optimize the assay for large-scale production, ensuring batch-to-batch consistency and rigorous in-process/final product QC tests. Our goal: every kit meets the highest performance and reliability standards.
Published Data
1. Development of a Novel Immunoassay for Presepsin (sCD14-ST) Detection
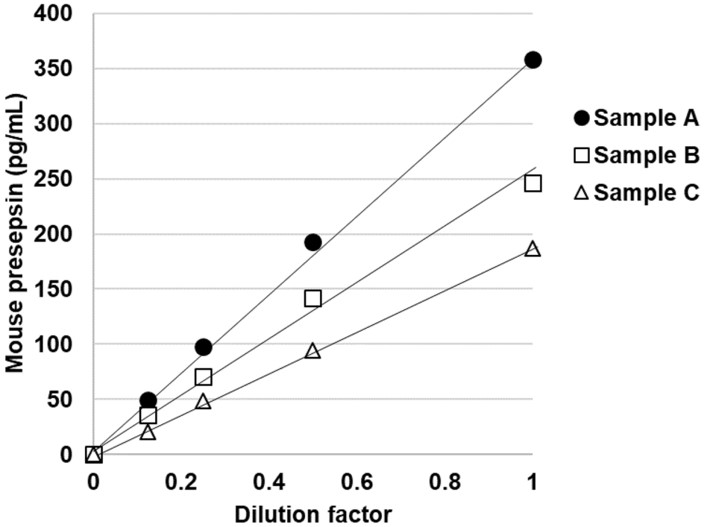 Fig.1 Linearity of the developed ELISA for mouse presepsin.1,3
Fig.1 Linearity of the developed ELISA for mouse presepsin.1,3
This study presented a sandwich enzyme-linked immunosorbent assay (ELISA) with high sensitivity for detecting presepsin in mouse plasma, designed to explore its association with diseases. Polyclonal antibodies were generated from rabbit antiserum immunized with peptides, and recombinant mouse presepsin-Fc fusion protein was expressed and purified for use as a standard. The method’s linear detection range was 4.7–300 pg/mL, with a detection limit of 1.4 pg/mL. The assay specifically detected mouse presepsin without cross-reactivity from soluble CD14 (sCD14), even after digestion by cathepsin D proteinase. This method provides high specificity and is valuable for investigating presepsin-related diseases.
2. Development of Lateral Flow Immunoassay with a Stacking Pad Design
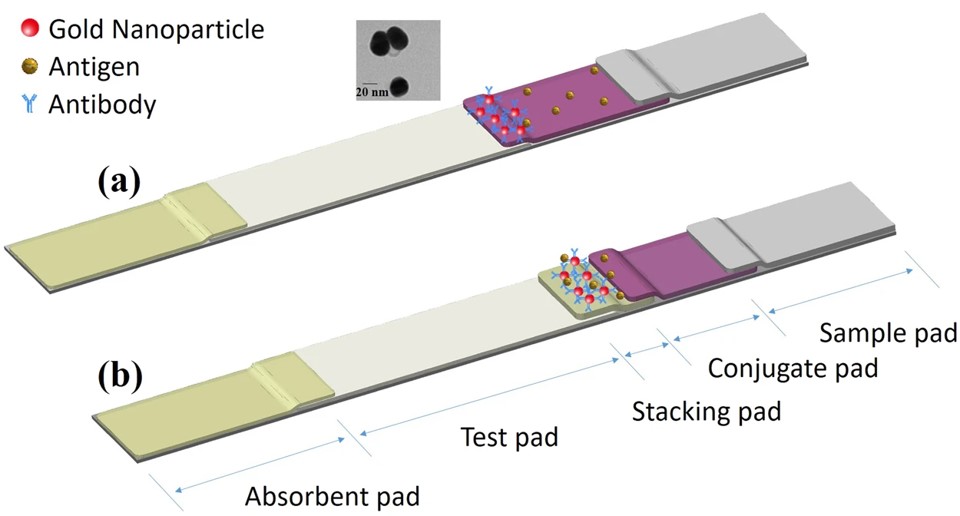 Fig.2 Proposed mechanism of the LFIA and sLFIA tests.2,3
Fig.2 Proposed mechanism of the LFIA and sLFIA tests.2,3
In this study, researchers developed an enhanced-sensitivity lateral flow immunoassay (sLFIA) without requiring additional steps or complex signal amplification. The method used a “stacking pad” configuration, which added an extra membrane between the conjugation pad and test pad in the conventional AuNP-based LFIA format. This design accumulates antibodies and antigens on the stacking pad, enhancing antigen/antibody binding interactions and improving detection sensitivity. The assay can detect as low as 1 ng/mL of Protein A and 15.5 ng/mL of C-reactive protein with the naked eye. It was successfully applied to analyze C-reactive protein in human serum and synovial fluid, offering a sensitive, on-site diagnostic tool for resource-limited settings.
Why Working with Creative Biolabs?
-
Flexibility
Whether you need a one-time product or service, a combination of products and services, or a fully customized antibody/kit development program, we are ready to put our expertise and technologies to work for you. Antibody development or immunoassay development for both research and diagnostic use. -
Expertise
Specialized in the production of high-quality antibodies and IVDs that detect a broad range of markers, including hormone, tumor markers, food safety, infectious organisms, etc. Abundant expertise with know-how in all steps of the research, development, manufacture, and assembly process. -
Support
Quick response to your needs from the first step to the last by offering individualized technical support to our valued customers by learning and understanding their objectives and doing our best to accommodate them accordingly. -
Value
Cutting edge technologies, keen project, and program management skills, flexibility, and a client-focused approach enable us to provide in-depth expertise to tackle the obstacles challenging the clients' business.
Q&A
-
Q: How do you ensure the quality of raw materials for my IVD immunoassay?
A: We implement a rigorous raw material qualification process that includes thorough vendor audits, comprehensive incoming quality control testing, and detailed material specifications. Our scientific team meticulously evaluates antibodies, antigens, and other critical components for purity, activity, stability, and lot-to-lot consistency to ensure they meet the stringent requirements for diagnostic applications.
-
Q: What types of samples can your IVD immunoassays be developed for?
A: Our knowledge covers a wide spectrum of biological matrices. We commonly develop assays for serum, plasma, urine, saliva, cerebrospinal fluid (CSF), and various tissue lysates. We work closely with clients to understand their specific sample requirements and ensure the assay is optimized for reliable performance within the intended matrix.
-
Q: How do you manage intellectual property and confidentiality for custom development projects?
A: We prioritize the protection of your intellectual property and private information. All projects are conducted under strict confidentiality agreements (CDAs), and our internal processes are designed to safeguard your proprietary data. We work with total openness and ensure that your innovations remain securely yours.
-
Q: What happens after the assay development is complete? Do you offer manufacturing support?
A: Yes, our service extends beyond development. We provide comprehensive manufacturing transfer support, including the creation of detailed SOPs and QC protocols necessary for scaling up production. We can also assist in identifying and vetting contract manufacturing organizations (CMOs) to ensure a smooth transition from development to commercial-scale manufacturing.
-
Q: Can I get support for only specific phases of the development workflow?
A: Absolutely. Our services are highly flexible. While we offer full, end-to-end development, clients can engage us for specific phases where they require specialized expertise, such as challenging reagent development, complex analytical validation, or assistance with specific regulatory documentation. We personalize our support to your specific needs.
-
Q: How do you ensure the long-term stability and reproducibility of the developed assay?
A: Long-term stability and reproducibility are built into our development process from the inception. We carry out accelerated and real-time stability studies on reagents and the final assay. Our optimization includes robust buffer systems, appropriate conjugation chemistries, and stringent manufacturing controls to ensure consistent performance over the product's shelf life and across different production lots.
Creative Biolabs has successfully developed a wide variety of immunoassays targeting different disease biomarkers. Bringing in years of experience and expertise, our dedicated team of professionals will help you with every step of the development process, thereby accelerating and bringing your immunoassay to market rapidly. Contact us to discuss your project and experience the great value of our services.
For Research Use Only.