In Vitro SSP Study on Gastrointestinal System
To meet the challenging requirements for drug assessment, Creative Biolabs has built a team of experienced scientists with facilities and processes designed specifically to provide the best strategy and protocols customized to drug assessment services. We have successfully accomplished a series of projects for identifying the efficacy and safety of a potential drug in a range of therapeutic areas.
Introduction to Gastrointestinal System
The gastrointestinal tract, also known as the gastrointestinal system or GIT, has been proved to be critical to the delivery of nutrients in human. GIT is a muscular tube that starts from the oral cavity, oesophagus to the anus. Previous studies have revealed that GIT can secret various enzymes and plays an important role in transforming food into components. Meanwhile, recent researchers have demonstrated that GIT is associated with a wide variety of accessory organs, such as liver, gall bladder. Furthermore, pilot studies have suggested that the abnormal function or inflammation of GIT will cause a range of diseases. However, no available methods have been proved and widely used for the diagnosis of GIT diseases. In addition, several studies have shown that a variety of drugs can induce the damage and dysfunction of GIT. GIT screening should be necessary for assessing the safety of candidate drugs in preclinical development. As a result, a series of technologies have been widely used for investigating the pharmacological safety of drug candidates on the gastrointestinal system.
Services of In Vitro SSP Studies on Gastrointestinal System
Currently, Creative Biolabs has established a panel of platforms to estimate the drug effects on the gastrointestinal system. We have successfully accomplished a series of in vitro GIT testing services based on cells, tissues as well as organoids to evaluate the mechanism of action (MOA) of potential drugs. In general, the gastrointestinal (GI) motility, gastric secretion and colonic function will also be analyzed in our platform, which could provide a strong basis for the safety of drugs. Moreover, we have developed multiple in vitro models for investigating the gastrointestinal absorption of candidate drugs. Additionally, we can also offer the services to examine the side effect, off-target interaction, and physiological or pharmacological responses by a variety of assays, including but not limited to, ligand binding assay, cell and enzyme analysis.
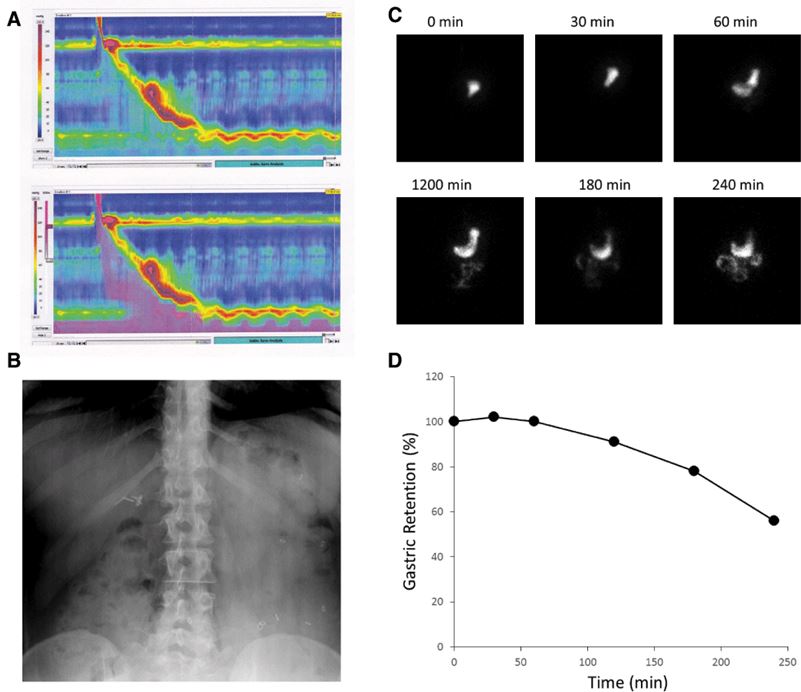 Fig.2 Examples of clinically used assessments of gastrointestinal (GI) motility.1, 2
Fig.2 Examples of clinically used assessments of gastrointestinal (GI) motility.1, 2
With years of operational experience, Creative Biolabs is able to provide the state-of-art services for any drug discovery and evaluation project. Our scientists specialized in GIT safety pharmacology studies will work with you to develop a most appropriate strategy that will offer the reproducible data for your research. Now, we have developed efficient protocols for drug safety assessment. And we can also conduct custom in vitro studies to meet any requirement of our clients. If you have any special needs in our drug discovery and evaluation services or be interested in learning more about our company, please feel free to contact us for more details.
Reference
- Klaus, B.; et al. Disorders of gastrointestinal hypomotility. F100Research. 2016, 5: 1987. under Open Access license CC BY 4.0, without modification.
For Research Use Only.
