Drug Abuse Liability
Creative Biolabs provides experiment design on a case-by-case basis and works closely with our customers to deliver the most effective and flexible abuse liability services tailored to your needs.
Drug abuse liability is the potential that a drug has properties that facilitate people becoming addicted or dependent on it. This phenomenon may be caused by drugs' pharmacological and psychopharmacological properties or social aspects around their self-administration. The addictive potency of drugs varies between different drugs. For instance, drugs such as heroin or cocaine have higher abuse liability than drugs like codeine or alcohol.
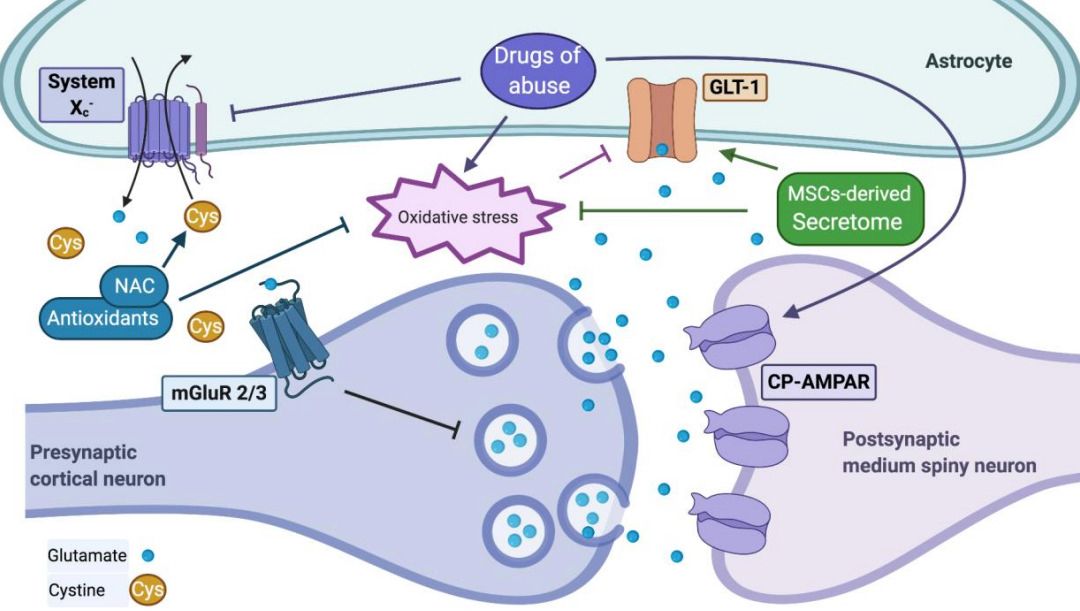 Fig. 1 Drugs of abuse modify the extracellular levels of glutamate. 1
Fig. 1 Drugs of abuse modify the extracellular levels of glutamate. 1
Abuse liability tests are a critical piece of information for investigational new drugs enabling. Abuse liability tests are responsible for protecting and improving public health, have the potential ability to find novel targets, and make drug discovery process avoid project delay or termination. The tests should be conducted on laboratory animals and humans to determine if the drug is addictive and if certain ways of administration make it more addictive. With our experience and knowledge, we are able to provide you the optimized abuse liability determination strategy and deliver you complete information for your drug discovery success.
Creative Biolabs offers a variety of approaches to assess the abuse potential of a drug product.
Evaluation of Chemical Similarity
We provide compound characterization services, including structure characterization, physicochemical characterization, and mode of action (MOA) determination to compare your chemical compounds to drugs leading to drug abuse, such as heroin or cocaine.
In Vitro Receptor Binding Studies
We conduct in vitro binding studies to determine the mode of action of the drug (binding to receptor, transporter, ion-gated channel system, etc.). A drug may have direct and indirect effects on neurotransmitter systems associated with abuse potential.
Animal Behavioral Pharmacology Studies
We follow the recommendations address the conduction of animal abuse potential studies guided by FDA. We offer self-administration, conditioned place preference, drug discrimination, psychomotor and dependence potential tests on several species of animals.
Pharmacokinetics/Pharmacodynamics (PK/PD) Properties
We assess data of maximum concentration (Cmax), time to maximum concentration (Tmax), the area under the curve (AUC0-∞), the terminal elimination half-life (T½), bioavailability, distribution volume (Vz), and clearance (CL) to establish PK/PD relationship. The information will be of value for correlating psychoactive drug effects with achieved plasma concentrations.
All of our abuse liability studies are performed in compliance with the US Food and Drug Administration (FDA) good laboratory practice (GLP) principles for safety pharmacology studies and in FDA regulations apply to abuse potential studies in animals to ensure quality and reliability of animal safety studies.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
- Berríos-Cárcamo, Pablo et al. "Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules." Antioxidants (Basel, Switzerland) vol. 9,9 830. 4 Sep. 2020, doi:10.3390/antiox9090830. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
