Pharmacology & Pharmacodynamics Study Services
Introduction to Pharmacology and Pharmacodynamics Studies
Pharmacology and Pharmacodynamics (PD) studies are critical components of drug development, providing essential insights into how drugs interact with biological systems and produce their effects. These investigations are essential for elucidating drug mechanisms of action, establishing dose-response relationships to optimize therapeutic efficacy and safety, identifying relevant biomarkers, guiding drug candidate selection, fulfilling regulatory requirements, and predicting clinical outcomes. Creative Biolabs has a professional scientific research team, advanced technology platforms, and many years of rich experience in preclinical disease model construction and evaluation. We can provide comprehensive pharmacology and pharmacodynamics services to our global customers, significantly accelerating your drug development process.
Available In Vivo Disease Models
Creative Biolabs has developed well-established animal models spanning multiple disease systems, including:
| Tumor Models | Neurological Disorder Models | Inflammation & Immunological Disease Models |
| Metabolic/Liver Disease Models | Urological System Disease Models | Digestive System Disease Models |
| Cardiovascular Disease Models | Ocular Disease Models | Musculoskeletal Disease Models |
| Respiratory Disease Models | Rare & Orphan Disease Models | Infectious Disease Animal Models |
| Reproductive System Disease Models | Ear Disorder Models | Skin Disease Models |
In Vitro Pharmacodynamic
We offer comprehensive pharmacology and pharmacodynamics study services that span the entire spectrum from initial discovery to preclinical development, encompassing both in vitro and in vivo evaluations. Our in vitro services focus on high-precision, mechanistic studies conducted in controlled laboratory settings (cell-free systems, isolated cells, or tissue cultures), including but not limited to:
Evaluation Platform
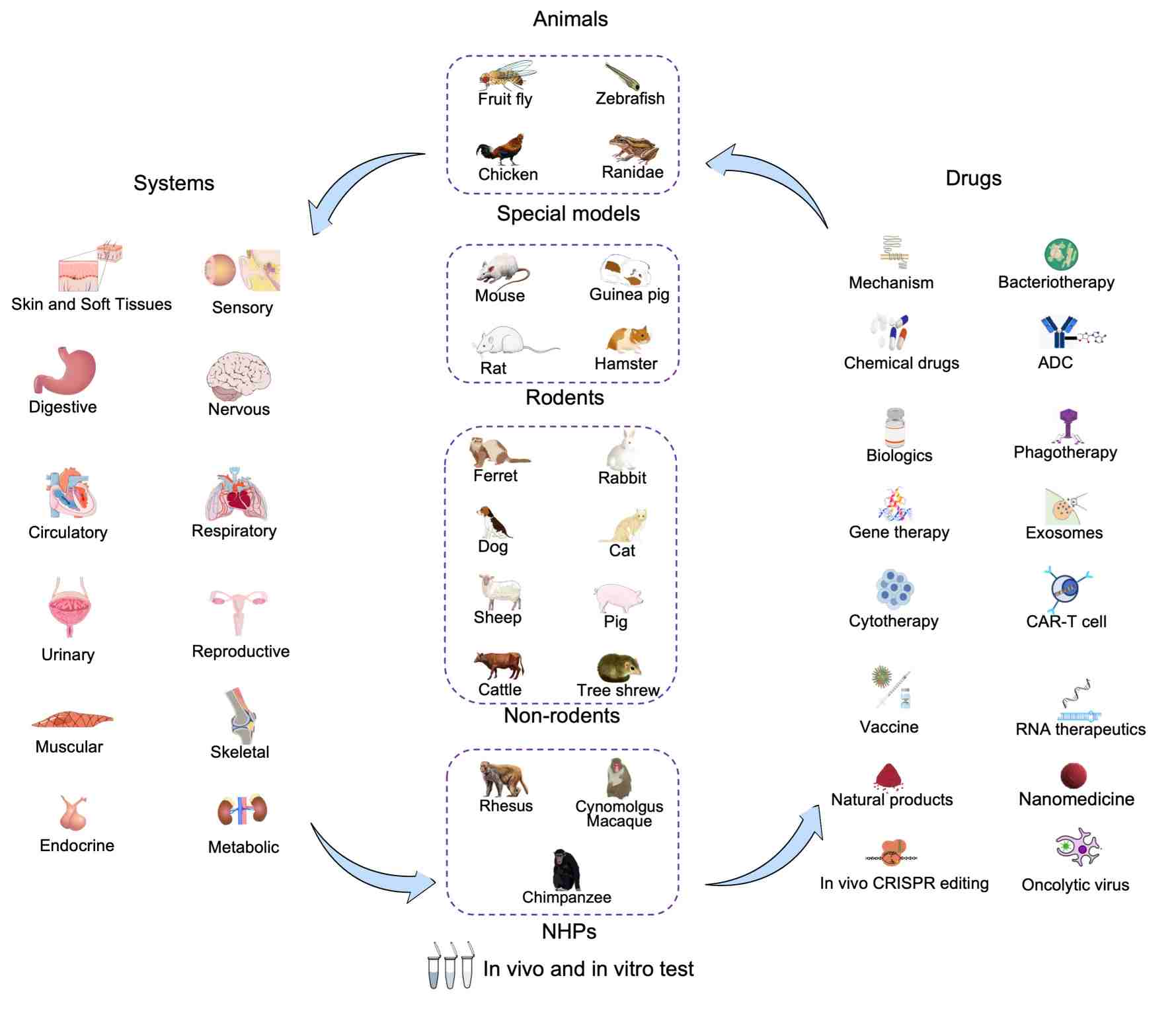
Creative Biolabs has established disease models across multiple therapeutic areas, supported by validated models in diverse species.
Developed Therapeutic Areas
Respiratory system diseases, digestive system diseases, nervous system diseases, endocrine system diseases, urinary system diseases, reproductive system diseases, circulatory system diseases, immune system diseases, sensory system diseases, metabolic system diseases, musculoskeletal system diseases, skin and soft tissue diseases, infectious diseases, genetic diseases, oncology, trauma and poisoning, aging-related diseases, and rare diseases.
Available Animals used for In Vivo test
- Rodents: such as mouse, rat, hamster, mongolian gerbil, cotton rat.
- Non-rodents: such as rabbit, dog, miniature pig, ferret, mink, sheep, cattle, horse, tree shrew.
- Non-Human Primates (NHPs): such as rhesus macaque, cynomolgus macaque, marmoset.
- Other special species: such as fruit fly, chicken, zebrafish.
- Featured models: humanized PDX models, gene-edited animals (CRISPR customization).
Related In Vitro Analysis
Drug screening, cell proliferation/toxicity, ion channel analysis, immune cell phenotyping/differentiation, biomarker analysis, blood biochemistry analysis, cytokines or chemokines test, cell signaling pathways analysis, behavioral studies, pathology evaluate, complete blood count (CBC) immunofluorescence staining, in vivo imaging, reporter gene cells, gene transfection, immunocyte co-culture killing assay, CRISPR/Cas9 gene editing, RNA interference (RNAi), high-content screening (HCS), and transgenic animals.
Currently Available Instruments
Creative Biolabs' services include several detection platforms featuring state-of-the-art analytical equipment, such as:
| Platform | Equipment |
|---|---|
| Cell Biology | CO2 incubator, Live cell workstation, Biosafety Cabinets, Microinjection apparatus |
| Molecular Biology | Bio-Rad QX200, Next-generation sequencing (NGS), Gel electrophoresis systems, Nucleic Acid Electrophoresis and Blotting, Electroporator |
| Pathology | Automated Slide Stainers, Microtomes, Cryostats, Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Digital pathology scanners |
| Immunology | ELISA Reader, Meso Scale Discovery (MSD), Confocal microscopy, Flow cytometry, Luminex Instrument, ELISPOT Reader |
| Hematology | Automated biochemical analyzer (Roche Cobas c501), High-Performance Liquid Chromatography (HPLC), Blood gas analyzer, Coagulation analyzer, Blood Rheometer, Liquid Chromatography-Tandem Mass Spectrometry System (LC-MS/MS) |
| Behavioral Studies | Open Field Test (OFT), Morris Water Maze (MWM), Elevated Plus Maze (EPM), Gait Analysis Systems, Automated Activity Monitors, Water Maze, Rotarod performance test, Operant Conditioning Chamber, Video Tracking Software, Metabolic Cage |
| Imaging | Ultrasound imaging, Micro-computed tomography (micro-CT), Magnetic resonance microscopy, small animal PET∕SPECT∕CT imaging system, Bioluminescence Imaging (BLI) |
Applications
Creative Biolabs' platforms are designed to support diverse research and development activities, ensuring comprehensive and integrated solutions for client projects. We have accumulated extensive drug validation experience, including:
- Small Molecules: Natural products, Chemicals, Molecular Glues, Enzyme inhibitor, Receptor agonists or antagonists, Ion channel regulators, metabolic modulator, Targeted covalent inhibitors (TCIs), PROTAC technology.
- Large Molecules: Cytokines, Fusion proteins, Monoclonal antibody (mAb), Bispecific antibody (BsAb), Polyclonal antibody (pAb), Antibody-Drug Conjugates (ADCs), enzyme therapeutics, hormone proteins, peptide therapeutics.
- Cell Therapy: Stem cells, induced pluripotent stem cells (iPSCs), CAR-T, CAR-NK.
- Gene Therapy: Antisense oligonucleotides (ASO), small interfering RNA (siRNA), Aptamers, in vivo CRISPR editing.
- Drug Delivery Systems: Nanoparticles, adenovirus vectors, lentivirus vectors, liposomes.
- Vaccines: Attenuated vaccines, inactivated vaccines, mRNA vaccines, recombinant protein vaccines.
- Novel Therapies: Bacterial therapy, phage therapy, oncolytic viruses.
Our Advantages
1. Rich Animal Resources and Comprehensive Disease Model Construction Capability
Creative Biolabs' animal models cover over 10 types of experimental animals, including mice, rats, rabbits, dogs, and non-human primates, meeting the needs of different drug development stages for animal models. Based on this, we have the capability to construct and evaluate comprehensive system disease models, covering fields such as oncology, the nervous system, cardiovascular, and metabolism. For example, in cancer research, we can construct not only conventional models like subcutaneous tumors and orthotopic tumors but also customize genetically engineered tumor models. For neurological diseases, we possess established models for Alzheimer's and Parkinson's diseases and ensure the validity of these models through multi-dimensional evaluation systems, such as behavioral and imaging assessments, providing a precise in vivo research foundation for drug development.
2. One-Stop In Vivo and In Vitro Service System
Creative Biolabs has developed a complete closed-loop in vivo and in vitro research service, efficiently covering everything from in vitro cell experiments and molecular mechanism validation to in vivo animal pharmacological evaluation and pharmacokinetic analysis within the company. Clients do not need to coordinate with multiple agencies, thereby reducing communication costs and time loss. Taking innovative drug development as an example, clients can first conduct target screening and preliminary activity validation through our cellular platform, then proceed to efficacy evaluation and safety assessment using the animal platform, and finally complete pharmacokinetic parameter determination through the drug analysis platform, ensuring seamless integration throughout and significantly enhancing research and development efficiency, thus facilitating rapid project advancement.
3. Professional Team and Comprehensive Management System
Creative Biolabs unites experts from diverse fields, including pharmacology, toxicology, and biomedical sciences, with key members boasting decades of industry experience. An independent Quality Assurance (QA) department has been established to oversee and review all aspects, from experimental design and operational procedures to data documentation. The high reliability of our experimental data guarantees that we deliver high-quality, dependable research results to our clients. This foundation makes us a robust partner for long-term collaboration, supported by our professional expertise and regulated management practices.
4. Multi-Omics Integration Analysis
Creative Biolabs combines transcriptomics, proteomics, metabolomics, and other omics technologies to comprehensively elucidate drug mechanisms of action at the molecular level, thereby providing a more complete theoretical basis for drug development.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1. Does the company's detection equipment have data traceability and audit functionality?
A1: To maintain its technological leadership, Creative Biolabs utilizes advanced, comprehensive detection instrumentation sourced from internationally renowned manufacturers. All key instruments are equipped with integrated electronic data recording systems that enable real-time acquisition, storage, and backup of experimental data. These systems provide full data traceability and robust audit trail capabilities, meticulously logging data generation, modification, and access. This rigorous approach ensures data authenticity, integrity, and traceability, ensuring compliance with regulations and satisfying client audit requirements.
-
Q2. What technical service platforms has the company established?
A2: Creative Biolabs has established comprehensive Pharmacology and Toxicology platforms spanning Molecular biology, Immunology and Cell Biology, Histopathology, Animal Model, Data Analysis and Bioinformatics Platforms. For instance, our molecular biology platform supports experiments such as gene cloning, qPCR, and Western blotting. The animal model platform can establish various disease models, including those for cancers, neurological disorders, and cardiovascular diseases. Furthermore, our drug analysis platform provides research services for Pharmacokinetics and Toxicology. This integrated approach allows us to offer clients one-stop technical solutions.
-
Q3. Can customized technical services be provided?
A3: Yes. Creative Biolabs has a professional technical R&D team that can customize personalized experimental models based on the special needs and research goals of the clients (such as drug mechanism, dose-effect relationship, target validation) by integrating cutting-edge research technologies. From experimental design and technical route optimization to data interpretation, the team will communicate and collaborate with clients throughout the process to ensure the smooth implementation of projects and achieve the expected goals.
-
Q4: How to address species-specific differences in humanized antibodies in rodents?
A4: Addressing species-specific differences in humanized antibodies efficacy in rodents is crucial. We offer primary solutions:
- Humanized Mouse Models or Transgenic Mice Expressing Human Genes: Such as utilize huPBMC or CD34+ humanized mouse models, which incorporate human immune system components.
- Pharmacologically Relevant Species: such as Recommend Non-Human Primate (NHP) models for pharmacodynamic evaluation, as they offer closer physiological and immunological parallels to humans.
- Antibody Engineering: For studies in standard rodent disease models, a humanized antibody can be "murinized."
By integrating these strategies, researchers can obtain more translatable preclinical data for humanized antibodies, accelerating their development.
Reference
- The icons presented in Figure 1 are sourced from Scientific Drawings and Wikimedia Commons. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
