Screening Immunogenicity of Predicted T-Cell Epitope
Immunogenicity refers to the ability to stimulate the body's immune system to form specific antibodies or sensitized lymphocytes. The assessment of immunogenicity is closely related to the development of biopharmaceuticals. Proteins, antibodies, coupled peptides, and oligonucleotides involved in biotherapeutics may be highly immunogenic and induce harmful immune responses in the body, posing a serious threat to human health.
It has been reported that T cell epitopes and drug immunogenicity is inextricably linked. Therefore, the prediction of T cell epitopes and the screening of drug immunogenicity are indispensable, which is also a hot spot in drug research today. With years of experience in immunogenic screening research, Creative Biolabs has built a series of advanced instruments and database platforms to provide one-stop, customized T cell epitope prediction services for worldwide clients.
Why do We Screen Immunogenicity?
- Immunogenicity affects the safety and efficacy of drugs and even causes a deadly new disease caused by the immune reaction and the crossover of endogenous proteins, leading to both human and financial resources.
- High immunogenicity results in a significant increase in development risk and loss of patients. More importantly, current prediction methods are not ideal. When the problem of immunogenicity is discovered in the third phase of clinical trials, the loss is heavy.
- Immunogenicity is a top priority for the FDA. All biopharmaceuticals must be evaluated for immunogenicity to ensure the safety and efficacy of drugs.
Screening Methods & Services at Creative Biolabs
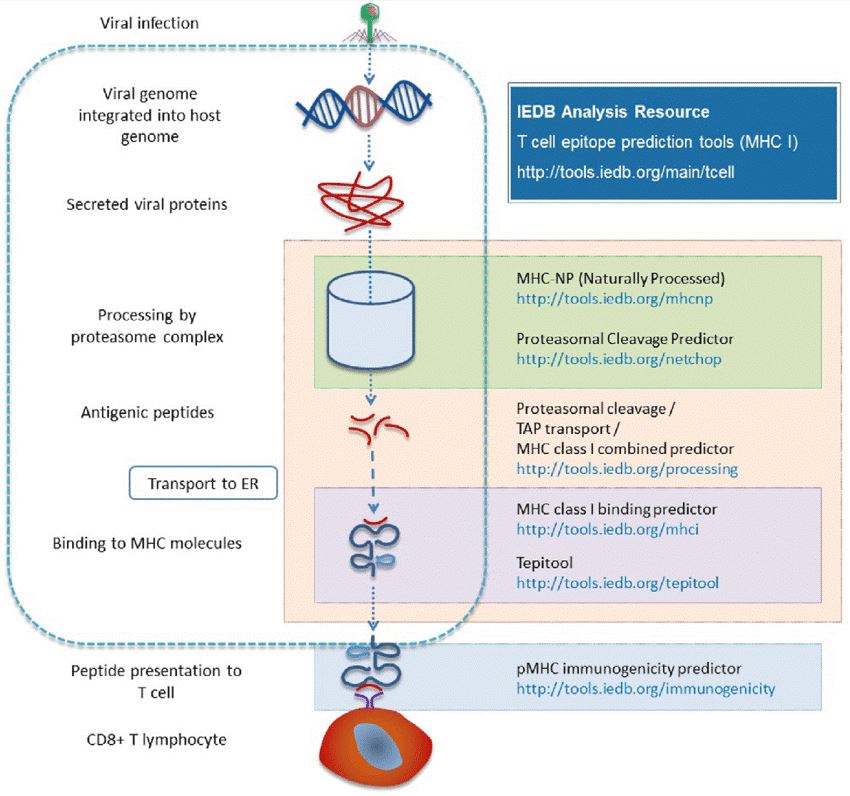 Fig. 1 Different prediction tools are available in the Analysis Resource with respect to different stages of major histocompatibility complex (MHC) antigen processing.1
Fig. 1 Different prediction tools are available in the Analysis Resource with respect to different stages of major histocompatibility complex (MHC) antigen processing.1
Typically, the body presents T cell epitopes to T cells to activate the production of drug antibodies. A number of experimental assays and computer simulation software or platforms have been developed for screening immunogenicity. Before entering clinical studies, Creative Biolabs' researchers can screen for the immunogenicity of drugs by predicting T cell epitopes on a computer and validating them in vivo and in vitro. In addition, this part of technology is also widely used in vaccine design and autoimmune screening. To date, our research methods and technical services including but not limited to:
Drug In Vitro Assays
- HLA stabilization assay
- T-cell sensitization experiments
- APCs assay
- Two-dimensional electrophoresis (2-DE) separation technology
- Neutralizing antibody technology
- Amino acid site-directed mutagenesis
- Other in silico screening
- T cell activation assays
- Statistical (additive) methods based on inference
- Non-additive methods based on inference
- Structure-based methods
- Enzyme-linked immunospot (elispot)
- Binding assays
- Mass spectroscopy (MS)
- Ex vivo immunogenicity testing
In Vivo Assays (Transgenic Mice)
Creative Biolabs provides many different animal models for in vivo assays, including MHC I (HLA-A2 ) transgenic mice, MHC II (HLA-DR4 and HLA-DR1) transgenic mice, MHC I + MHC II transgenic mice, and so on. For example, we typically use HLA transgenic mice and exposes them to proteins or other epitope peptides. These inbred mouse models, protein transgenic mouse models and other non-humanized animal models have been used to assess the immunogenicity of drugs and have achieved experimental success.
Our Highlights
- High requirements: perfect laboratory configuration, in line with national quality standards.
- High quality: match, analyze, and predict with multiple databases to deliver quality data analysis.
- One-stop service: a comprehensive design covering from column preparation, drug entrapment, immunogenicity screening to T cell epitope prediction.
With a high reputation in target identification, Creative Biolabs offers a full range of immunogenic screening and T cell epitope prediction services to reduce the immune response of drugs to the body. If you’re interested in our immunogenicity screening services, please directly contact us for more details.
References
- Fleri, W.; et al. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Frontiers in Immunology. 2017, 8: 278. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
