Direct Isolation & Analysis of MHC associated Peptide
Accurate predictions, isolation, and analysis of T-cell epitopes (peptides presented on major histocompatibility complex (MHC)) would be useful for immunotherapies for cancer and autoimmune diseases, designing vaccines, and improvement of protein therapies. As an excellent pharmaceutical company and CRO service provider, Creative Biolabs has broad technological expertise in drug target discovery and pre-clinical drug development to support the pharmaceutical industry and related biomedical sciences research communities worldwide. Identification, isolation, and analysis of immunogenic T-cell epitopes (MHC-associated peptides) compose a significant portion of our repertoire.
Immunogenic Epitopes of Tumor-Associated Antigen (TAA)
In the process of T-cell-mediated cancer immunotherapy, the targeted TAAs, more precisely, T-cell epitopes contained in these tumor proteins determine the effectiveness of treatment. With the advances of sequencing and isolation, numerous TAA-derived epitopes have been identified by sequencing peptides eluted from purified MHC from the tumor cells of cancer patients. Amongst, reverse immunology approaches are a powerful means for the definition of unmodified, high-affinity MHC-binding peptides as potential T-cell epitopes. However, diversely posttranslational modification possibilities lead to a tremendous diversity of MHC peptides, making the isolation and analysis of T-cell epitopes an indispensable tool.
Aided by our advanced instruments, fit-for-purpose laboratories, and professional knowledge, we have launched a large portfolio of methods to provide customized and suitable solutions for MHC-associated peptides’ isolation & analysis.
-
Isolation by Immunoaffinity Chromatography
Immunoaffinity chromatography (IAC) is a specialized form of affinity chromatography, which utilizes an antibody as a ligand immobilized onto a solid support matrix to specifically isolate and purify proteins and protein complexes. IAC can be used for analysis of unmodified and/or post-translationally modified MHC-associated peptides; therefore, it has the central importance in MHC peptides isolation.
-
Isolation by Acid Elution of Cell Surfaces
Acid elution is another common method used for the identification and isolation of MHC-associated peptides. There are two acid elution techniques to extract MHC-associated peptides have been developed:
- Strong acid elution of MHC class I and II peptides from the whole-cell lysate using trifluoroacetic acid.
- Mild acid elution (MAE) of MHC class I peptides from the cell surface. MAE has been successfully employed to identify T-cell epitopes from melanoma cells.
After elution, the peptides are mostly concentrated on cation exchange or reversed-phase cartridges before they are further analyzed. Compared to IAC, acid elution shows some apparent advantages, such as simplicity, cost-effectiveness, and the outlook to obtain mainly cell surface MHC peptides that are the relevant part for T-cell recognition.
-
Fractionation and Sequence Analysis of Isolated MHC Peptides
Because of the high complexity of MHC peptide pools, as well as the low yield of peptide isolation, sequence analysis of MHC peptide pools is a colossal challenge. In modern medical immunology, liquid chromatography (LC), particularly reversed-phase (RP)-HPLC, is the most common method to purify MHC peptides according to their lipophilicity. After purification, mass spectrometer (MS) has been regarded as the gold standard for the analysis of MHC peptides because of its high sensitivity (in the sub-femtomole range) and accuracy (lower than 5 ppm). To sequence a maximum number of MHC peptides from one isolation, the peptides can be directly transferred to an MS from the RP-HPLC system for fragmentation analyses.
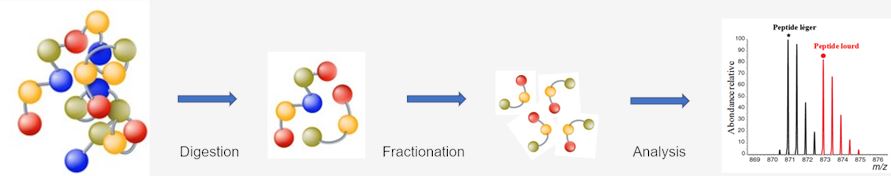 Fig.1 Workflow of fractionation and sequence analysis of MHC peptides at Creative Biolabs.
Fig.1 Workflow of fractionation and sequence analysis of MHC peptides at Creative Biolabs.
Identification, isolation, and analysis of MHC peptides are indispensable for gaining insight into the fundamental rules of immune recognition as well as for developing innovative immunotherapeutic approaches against cancer and other diseases. At Creative Biolabs, we are passionate about drug target discovery, offering comprehensive services across every stage of project development, including T-cell epitopes (MHC-associated peptides) isolation and analysis.
We strongly believe that the technological advances we make should be accessible to every customer. If you are interested in our services, please feel free to contact us.
For Research Use Only.
