All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Currently, there are several limitations of CAR-T cell therapy, including antigen escape, off-target effects, low CAR-T cell infiltration, immunosuppressive microenvironment, and CAR-T cell-related toxicity. While more critical obstacle to the widespread application of CAR-T therapy is the high price of CAR-T therapy. How to effectively engineer universal CART from autologous to allogeneic is the core. Engineering immune cells derived from induced pluripotent stem cells (iPSCs) offer great promise for developing CAR-mediated immunotherapy strategies for cancer and other diseases, which present several advantages, such as being more scalable, reproducible, cost-effective, and standardized. Using iPSCs as a starting source for CAR T development provides an attractive opportunity for cancer immunotherapy and facilitates CART product manufacture.
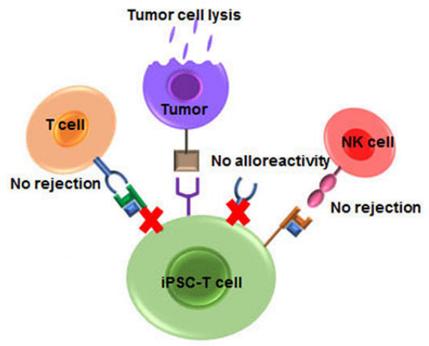 Fig.1 The immunotherapy properties of iPSC-T cells.1
Fig.1 The immunotherapy properties of iPSC-T cells.1
Characteristics of Human Induced Pluripotent Stem Cells (iPSCs)
Human induced pluripotent stem cells (iPSCs) are an ideal source of CAR-T cells for scalable manufacturing of cell therapies.
CAR-iT Cells Therapy Possessing Numerous Advantages
Compared with conventional CAR-T, the advantage of iPSCs-derived CART T-IPSC is based on the versatility character, and it does not require re-extraction of T cells for each patient. By obtaining cells from healthy donors in advance, it is expected to produce CAR-T cells that can treat hundreds of people at a time, which will dramatically reduce the cost of CAR-T production.
Generally, the generation of iPSC-derived CAR-T cells require three major processes.
Firstly, genetically engineering iPSCs to screen and preserve IPSC cell lines that can expand and differentiate into T cells.
Secondly, using the CRISPR/Cas9 technology to target the HLA (human leukocyte antigen) antigen on induced pluripotent stem cell-derived T cells (T-IPSC).
Finally, utilizing genetic engineering technology to incorporate an antigen receptor into iPSCs-derived T cells to recognize tumor cells.
These IPSC cell lines have been genetically engineered to differentiate into T cells that will not cause immune rejection in patients. When needed, they can be expanded to become a "universal" T-cell therapy.
CAR-iT Cells at Creative Biolabs
With years of experience in CART fields, Creative Biolabs is dedicated to delivering a full value chain offering for your CAR-iPSC project with advanced technology, comprehensive service, and professional expertise to accelerate your path to success. On the one hand, we provide several ready-to-use antigen-targeting CAR-iT cell products with strict quality control. On the other hand, we also provide special customized CAR products according to the customer's variable needs. Please feel free to contact us or inquire about an appropriate product or special solution for you.
Reference
Associated Antigen Target
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION