- Home
- ADC Development
- ADC In Vitro Analysis
- 3D Cell Culture based In Vitro Evaluation
- ADC Tumor Penetration
ADC Tumor Penetration
Tissue penetration efficiency is an essential parameter that should be taken into consideration for the development of any therapeutic agents including antibody-drug conjugates (ADCs). Generally, anti-cancer drugs are designed to be applied via intravenous infusion. Before reaching their desired target, drugs should firstly pass through the vascular wall, extravascular space, any intermediate cells or tissues, and sometimes certain barriers in the body. Specifically, there are diverse elimination systems or barriers, e.g., the blood brain barrier (BBB), blocking the way of ‘foreign’ components from reaching the inner parts of the body. Therefore, the tissue penetration capability of drug candidates should be high enough to pass though some of these barriers to take effect. Moreover, in terms of drugs aiming for solid tumor, they should also be capable to penetrate into the tumor tissue and achieve a lethal concentration in all tumor cells. The low tissue penetration ability of some anti-cancer drugs leads to the heterogeneous distribution of drugs inside the tumor microenvironment, which favors the rapid development of drug resistance.
For the development of anti-cancer drugs, especially those against solid tumors, the high tissue penetration capacity is a preferred property. A widely exploited approach to enhance the tissue penetration ability is the conjugation of drugs to a ‘helper’ moiety, such as tissue penetrating peptides, specific antibodies and other functional proteins, resulting in diverse bio-conjugates, with ADCs as a specific subfamily. The penetration efficacy of ADCs and a series of other drugs should be confirmed, assessed and optimized at the screening and optimization stages. This requires certain techniques and models for the analysis and estimation of ADCs before clinical trials. Traditional in vitro two-dimensional (2D) monolayer cells are not capable for the penetration analysis due to the lack of some important features when compared with in vivo tumor tissues, including the spatial tissue structure, cell-cell and cell-matrix interactions, as well as the influences of extracellular matrix and tumor microenvironments. Tumor-bearing animals are usually chosen for the pre-clinical in vivo assessment. However, it is costly and laborious to establish tumor-bearing animal models. Therefore, multiple design-evaluate-redesign cycles are needed and this can be a “bottleneck” in ADC developments, especially when the budget and the access to lab animals are limited. Besides, there are diverse uncontrollable factors that may interfere with the analysis. Due to the species gap between lab animals and human beings, the results collected by using lab animals may not be translatable into the clinical. Here comes the urgent need for a suitable bridge between in vitro 2D models and in vivo tumor analysis. Thus, three-dimensional (3D) cell culture techniques have become desirable for such purposes to analyze the penetration capabilities of ADCs.
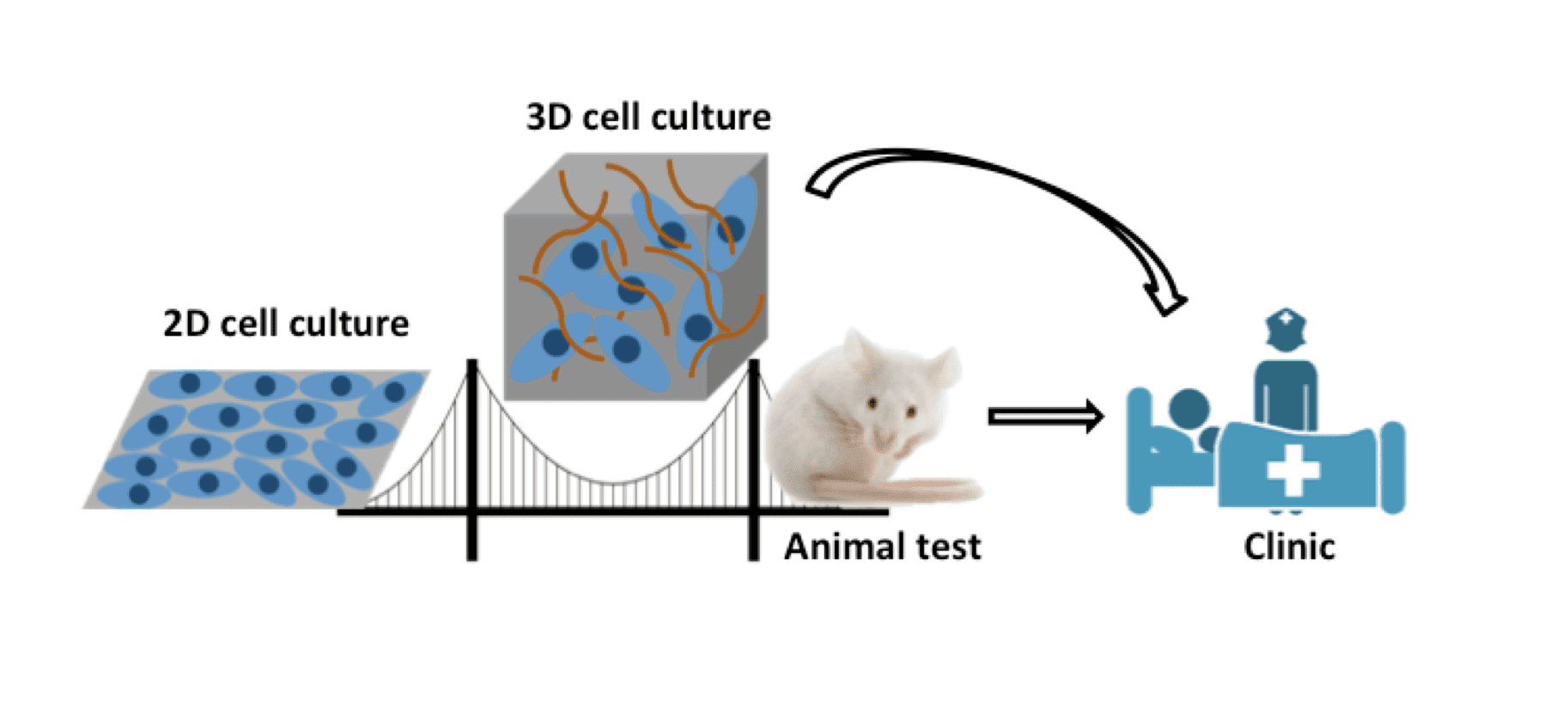 3D cell culture techniques can build up suitable bridges between in vitro 2D models and in vivo tumor tissues and favor the translation into the clinic.
3D cell culture techniques can build up suitable bridges between in vitro 2D models and in vivo tumor tissues and favor the translation into the clinic.
With the well-established 3D cell culture platform, Creative Biolabs provides straightforward in vitro penetration analysis for ADC products. At Creative Biolabs, we offer a wide range of tumor models in either static or fluidic systems, which can fulfill the versatile requirements for ADC penetration analysis. Our science team also helps customers with the establishment of other diseased tissue models, optimization of the analyzing protocols, as well as improvement and optimization of ADC products. With the expertise in ADC analysis and 3D cell culture, Creative Biolabs is dedicated to offer efficient and affordable analyzing strategies that significantly reduce the requirement for pre-clinical animal test. Please contact us for more information and a detailed quote.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
