- Home
- ADC Development
- ADC In Vivo Analysis
- ADC Safety Assessment
- Case Study: Safety Assessment for Mesothelin-targeting ADCs
Case Study: Safety Assessment for Mesothelin-targeting ADCs
Creative Biolabs fully understands the complexity of antibody drug conjugates (ADCs) studies. As part of our ADCs development programs, we offer comprehensive in vivo evaluation services including efficacy, PK/ADME, and toxicity analysis in various carcinoma models. In this section, we outline our efficacy and safety evaluation of mesothelin-specific ADCs as an example to demonstrate our in vivo analytical services.
Overview of Mesothelin
Mesothelin is a 40-kDa glycoprotein identified on mesothelial cell surfaces. Mesothelin expression has been reported in single layer mesothelial cells in pleura, peritoneum and pericardium. It is also actively expressed in surface epithelial cell in ovary, vaginalis, rete testis, and fallopian tube. The actual function mechanism of mesothelin remains unknown. However, a few hypotheses have suggested that mesothelin may contribute to cell adhesion. Several clinical studies have shown that the overexpression of cell surface mesothelin is linked to several human cancers, including epithelial mesotheliomas, lung cancer, and ovarian/pancreatic carcinoma. Collective data from various carcinoma studies has indicated a potential role of mesothelin as a therapeutic target for anti-cancer agents.
Currently, there is no commercially available mesothelin-targeting ADC products on the market. However, several candidates, including MDX-1204 and BAY 94-9343, are under active development in the pre-clinical stages. MDX-1204 is comprised of a human anti-mesothelin monoclonal antibody conjugated with duocarmycin, a DNA alkylating agent. In vivo animal experiments using mice and non-human primates have shown that MDX-1204 has tolerable toxicity and decent anti-tumor activities. BAY 94-9343, on the other hand, consist of a human anti-mesothelin IgG1 coupled with a tubulin-binding drug DM4. In vitro experiment results have shown that BAY 94-9343 exhibits promising inhibitory effects on mesothelin-expressing mesothelioma, ovarian and pancreatic cancer cell lines. A phase I clinical trial of this drug is ongoing at the moment to evaluate its potential toxicity.
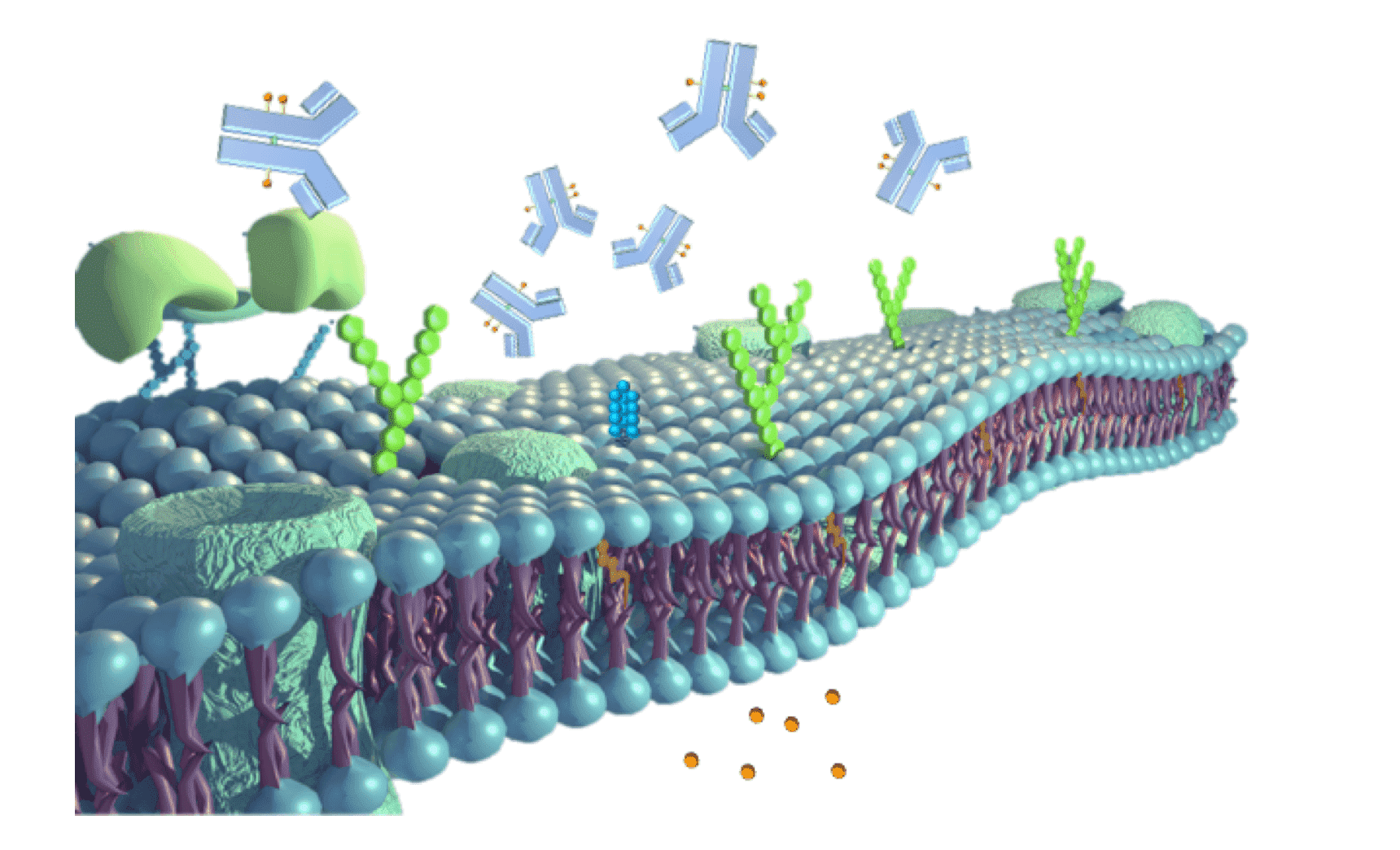 Presence of anti-mesothelin ADC on surface of normal cell and cause on-/off-target toxicity. On-target toxicity: binding of ADC to target-expressing normal cell. Off-target toxicity: non-specific binding to normal cell or released cytotoxins from instable ADC internalized by normal cells.
Presence of anti-mesothelin ADC on surface of normal cell and cause on-/off-target toxicity. On-target toxicity: binding of ADC to target-expressing normal cell. Off-target toxicity: non-specific binding to normal cell or released cytotoxins from instable ADC internalized by normal cells.
Mesothelin-expressing Tumor Model Establishment
To study the effects of ADCs on mesothelin-associate cancers, we offer both conventional tumor cell lines and tumor model established from patient-derived tumor cells for efficacy evaluation. Creative Biolabs is in possession of a large collection of human tumor cell lines. For mesothelin-positive tumor model development, we provide human pancreatic carcinoma PaCa-2, human colorectal cancer HT-29, human ovarian cancer OVCAR-3, and human squamous NCl-H226 cell lines as potential candidates.
-
Preparation of Mesothelin-expressing cells
Creative Biolabs develops mesothelin-positive tumor cells by viral transfection. The resulted cells are selected by real-time PCR and western blot. Similarly, for patient-derived tumor cells, expression level of mesothlin is confirmed by real-time PCR, western blot, and Immunohistochmical analysis.
-
Orthotopic and metastatic cancer model
To generate these models, immune-deficient mice are anesthetized during tumor incubation, after tumor implantation and incision closure. Tumor growth will be monitored and evaluated by bioluminescent imaging or necropsy analysis. Experienced technicians at Creative Biolabs have adequate surgical skills and all experiments are carried out following standard protocols approved by Institutional Animal Care and Use Committee (IACUC).
-
Subcutaneous xenograft model
In these models, immunocompromised mice receive subcutaneously adequate injections of mesothelin+ cells. Injection sites are usually located in the right or left flank. Depending on the injected cell amount, tumor cell will grow to meet criteria within days or weeks. Tumor size can be directly measured by vernier caliper.
-
Bioluminescent imaging
Creative Biolabs offers bioluminescent imaging system to monitor orthotopic and metastatic tumor cells. We develop firefly luciferase-expressed tumor cells and measure bioluminescent intensity after injection of D-luciferin. Results of this test can be confirmed in postmortem analysis.
-
Mesothelin-targeting ADC efficacy assessments
After model establishment, the in vivo efficacy of the mesothelin-targeting ADC is fully evaluated using similar strategies described in Case Study 1: Pharmacokinetics Analysis of HER2-targeting ADCs.
Safety Assessment of Mesothelin-specific ADCs
Creative Biolabs offers comprehensive toxicity tests for ADCs safety evaluation. We apply two animal species, usually rat and a non-human primate such as monkey, for this assessment to provide information such as dosage tolerance, tissue cross-reactivity, hemolytic potential….
-
ADC dosage tolerance
This parameter is measured by single dose and/or repeat does toxicity analysis. Generally, various doses of the tested ADC are administrated into animals at designed intervals and sample physiological changes, such as body weights, food consumption, clinical pathology, gross necropsy, organ weight… are carefully monitored and fully documented via clinical observations, physical examinations, and postmortem analysis. Unconjugated mAb and the payload drug are used as controls for assessing the overall impact of drug conjugation on the safety profile of the resulted ADC.
-
In vivo stability, metabolism, and clearance
Plasma concentrations of different ADC components (conjugated/unconjugated antibody, conjugated/free drug), the rate of ADC clearance, and final ADC metabolic products are measured using ELISA and LC/MS analysis to determine the status of the ADC in circulation.
-
Tissue cross-reactivity
ADC tissue cross-reactivity and the severity of ADC treatment on different organ and tissue samples are measured via immunohistochemical analysis using diverse animal and human bio-specimen from valid source.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
