- Home
- ADC Development
- ADC In Vivo Analysis
- ADC Pharmacokinetics Characterization
- Case Study 2: Tracing of Radiolabeled CD33-specific ADCs
Case Study 2: Tracing of Radiolabeled CD33-specific ADCs
Creative Biolabs fully understands the complexity of antibody-drug conjugates (ADCs) researches and we are dedicated to provide our clients with top-quality services and products for ADC development. To support pre-clinical ADC studies targeting CD33, we offer comprehensive in vivo evaluation services, including efficacy assessment in various carcinoma models, PK/ADME, and toxicity analysis, as part of our ADC development program.
Overview of CD33
CD33 (sialic acid-binding sialoadhesin receptor 3) is a 67-kDa glycoprotein that functions as a transmembrane receptor mostly expressed on myeloid cells as well as some monocytic precursor cells. In normal conditions, CD33 functions as an early stage myeloid differentiation antigen. Over-expression of CD33 is identified as biomarker of acute myeloid leukemia (AML), with about 85% of AML patients showing CD33 positive. In the meantime, this marker is virtually absent in normal cells, making CD33 a valuable anti-cancer target.
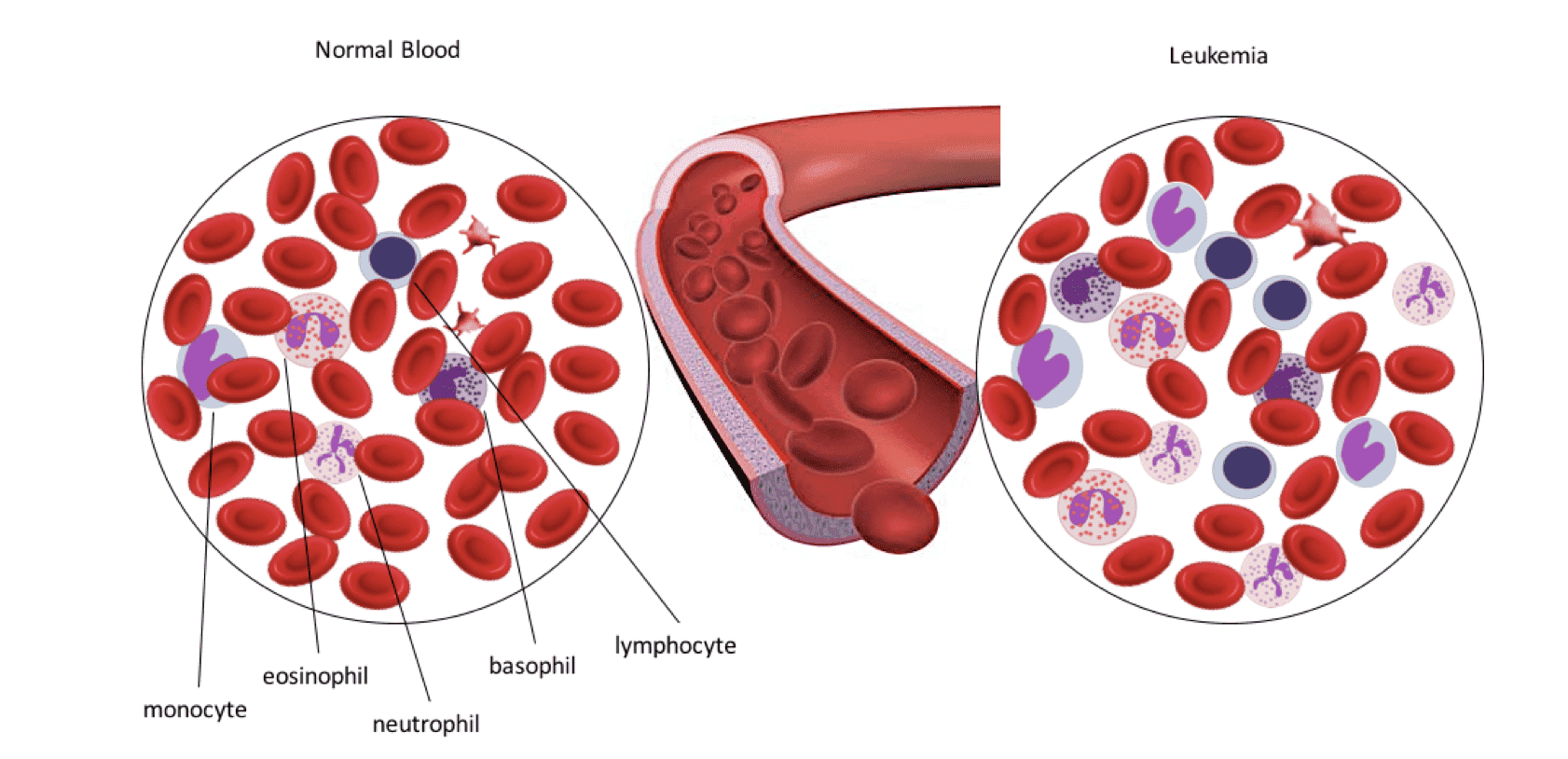 Fig.1 Comparison of normal blood and blood of patient with leukemia. Latter is characterized with abnormal number of leukocytes.
Fig.1 Comparison of normal blood and blood of patient with leukemia. Latter is characterized with abnormal number of leukocytes.
Anti-CD33 ADC therapy
A few ADC products have been released into the market for CD33-positive acute myeloid leukemia treatment. Gemtuzumab ozogamicin, consisting of a humanized anti-CD33 monoclonal antibody and the antitumor agent calicheamicin connected via butanoic acid linker, is among the first produced anti-AML recombinant drugs. Unfortunately, gemtuzumab ozogamicin was withdrew from the market due to increased mortality in clinical trials. More recently, a novel anti-CD33 ADC named vadastuximab talirine has been developed by attaching pyrrolobenzodiazepine dimer, a highly potent DNA crosslinking agent, to a monoclonal antibody.
In vivo Efficacy Evaluation of Anti-CD33 ADCs
The anti-CD33 ADC in vivo efficacy evaluation is divided into two stages:
(1) Efficacy evaluation by xenograft animal models:
Immuno-deficient mice from Creative Biolabs are housed in pathogen free environment and managed by professional veterinarian. Animal experiments are conducted in accordance with protocols reviewed by Institutional Animal Care and Use Committee (IACUC). Xenograft mice models are generated to assay the tumor inhibition efficacy of ADCs against CD33. AML cells such as U937, MV4-11, HL-60 or MOLM-14 are cultured and replicated in reported media, washed with PBS buffer, filtered through 0.2mm cell filter, and suspended in PBS medium. The cells are injected into mice via tail vein or implanted in right or left flank depend on cell line and mice strain used for tumor formation. Daily cage-side observations are conducted to record physiological change in animals. Signs of diseases, including ruffled coat, weakness, reduced motility, hunched back, etc., are carefully monitored and fully documented. Blood samples are taken following protocols and bone marrow samples are obtained after animal sacrifice. Burden of disease in blood and bone marrow is monitored by flow cytometric staining for human CD33+ cells.
(2) In vivo pharmacokinetics (PK) analysis:
Scientists at Creative Biolabs perform biochemical characterization analysis to evaluate the behavior of an ADC in vivo and to track its distribution via radio-isotope labeling.
-
ADC in vivo characterization:
Analytes tested to determine the ADC pharmacokinetics (PK) profile include the total antibody, drug-conjugated antibody, antibody-conjugated drug, and unconjugated drug. The bioanalytical team at Creative Biolabs offers ELISA and LC-MS/MS based methods to quantify these analytes. Please refer to Case Study 1: Pharmacokinetics Analysis of HER2-targeting ADCs for detailed information. -
Radiolabeled ADCs for bio-distribution study
Creative Biolabs’ bioanalytical group has extensive experience in radioactive tracer development and in vivo tests. All animals for experiment are quarantined for several days before initiation, and received radiolabeled ADC injections in appropriate amount. Following dose administration, bile is collected from duct-cannulated mice. Blood samples, animal urine, and feces are collected from non-cannulated mice at designed interval (varied based on project requirement). Animal tissues and organs are harvested at necropsy. Collected samples are processed and analyzed in liquid scintillation analyzer to determine the distribution profile of the assayed ADC. -
ADC catabolism studies
Complex catabolic pathways add additional complexity in ADC in vivo evaluation. The antibody portion and the drug undergo different catabolic pathways for their clearances. Elimination of the antibody portion is similar to that of a regular monoclonal antibody, which occurs either in lysosome via receptor-mediated endocytosis or as fluid-phase pinocytosis. Drug elimination mainly occurs in liver or spleen. Creative Biolabs conducts ADC catabolism study by using postmortem liver or commercial liver fraction (animal or human depend on project requirement). Liver fraction is prepared in appropriate buffer and reacts with ADC as substrate. Mixture is removed at different time-point, quenched and centrifuged to collect supernatant for LC-MS analysis. Postmortem liver sample is processed in similar method except digestion step.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
