- Home
- ADC Development
- DrugLnk™ Custom Linker-Payload Synthesis
- Drug Module Synthesis
- DNA Toxin Synthesis
- Bleomycin A2 Synthesis
Bleomycin A2 Synthesis Service
With the advanced "DrugLnk" organic synthesis platform and accumulative experience in toxin chemistry for antibody-drug conjugates (ADCs) development, Creative Biolabs provides highly customized ADC preparation services using bleomycin A2 and its derivatives as payloads.
Bleomycins, a group of glycopeptide antibiotics, were initially discovered from Streptomyces verticellus by Umezawa and co-workers in 1966. Bleomycins have been demonstrated to exhibit low immunogenicity and excellent tumor suppression activities, making them attractive anti-tumor therapeutics. However, the therapeutic effects of bleomycins are restricted by their dose-dependent pneumonitis. Bleomycin A2, one member of the bleomycin superfamily, is the main constituent (70%) of the clinical anti-cancer drug Blenoxane, which is utilized for the treatment of various cancer types, including Hodgkin’s lymphoma, testicular cancers, as well as carcinomas of the head, skin, and neck. Bleomycin A2, whose structure was solved by a series of chemical degradation analyses in combination with X-ray crystallography, shows a different structure from other naturally occurring bleomycins in the cationic C-terminus. Meanwhile, the relative and absolute stereochemistry of bleomycin A2 has also been characterized.
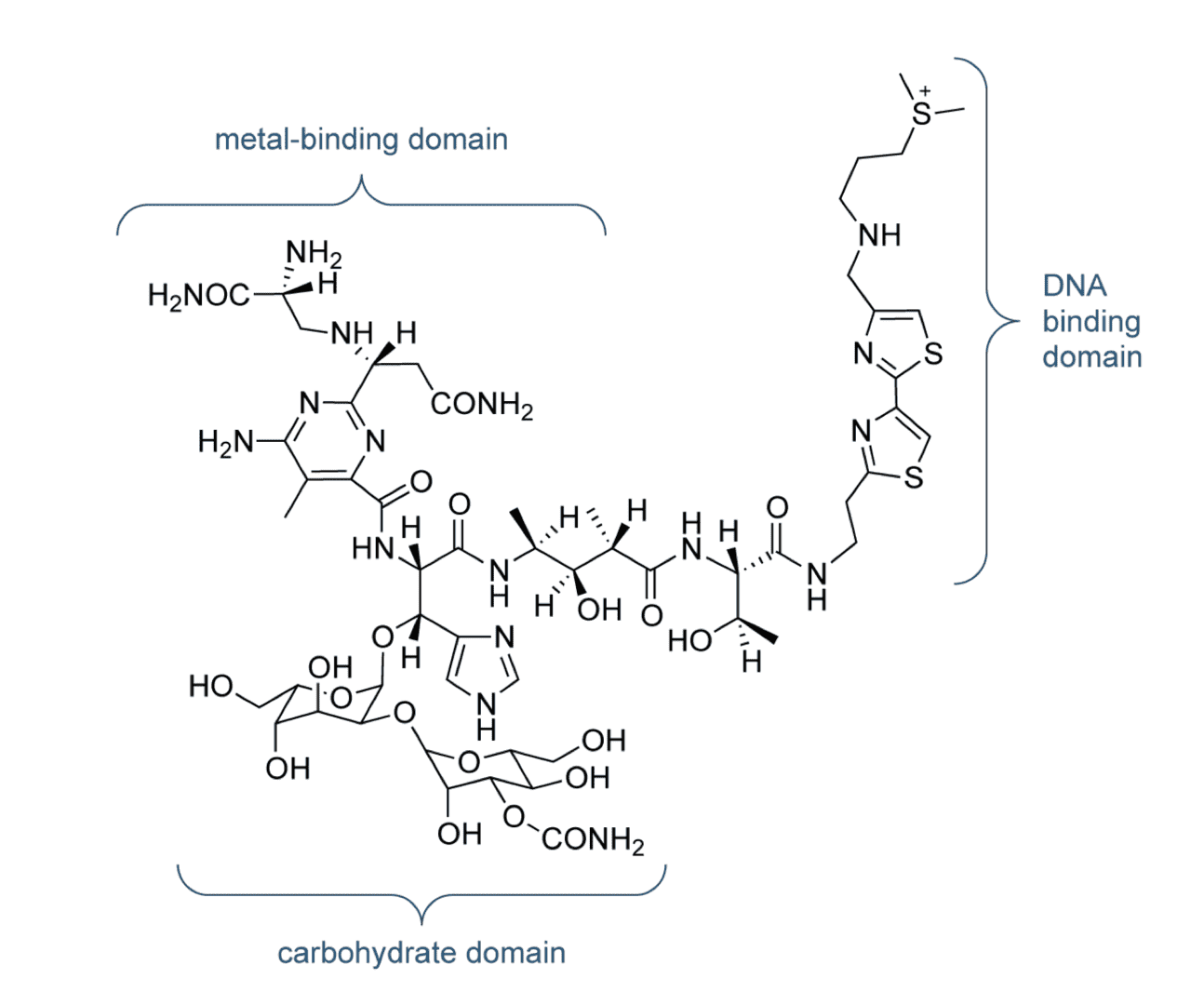 Fig.1 The chemical structure of bleomycin A2.1,3
Fig.1 The chemical structure of bleomycin A2.1,3
Bleomycin A2 Mode of Action (MOA)
Bleomycin A2 is proposed to carry out its biological function by binging and degrading DNA, a process which requires the presence of a metal ion and oxygen. Bleomycin A2 selectively cuts double-strand DNA at 5’-GC or 5’-GT sites through minor groove C4’-H atom abstraction and via fragmentation of the deoxyribose backbone. Both single- and double-stranded DNA cleavage have been observed from bleomycin A2 and the latter is more common. Recently, bleomycin A2 has been proven to cleave RNA and DNA-RNA hybrids, offering extra nucleic acid targets. From a structural point of view, bleomycin A2 can be divided into several functional domains that play essential roles in its overall biological activity. Previous studies on bleomycin A2 and its derivatives have showed that the N-terminal pyrimidine, comprised of a b-aminoalanine amide side chain and the linked β-hydroxy-L-histidine, is the metal binding domain in bleomycin A2 while the DNA binding domain resides at the cationic C-terminus.
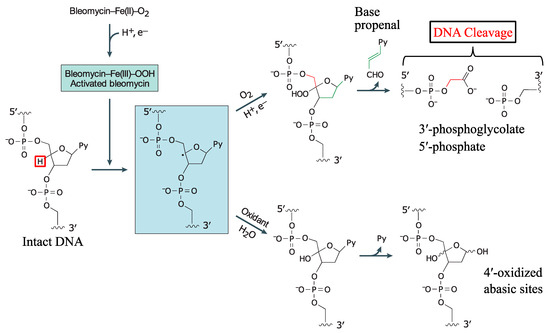 Fig.2 Model of bleomycin A2 bound to DNA.2,3
Fig.2 Model of bleomycin A2 bound to DNA.2,3
Bleomycin A2-based ADCs
ADCs are designed to specifically target and kill cancer cells by delivering highly potent toxic agents, namely payload, to the tumor sites. In order to develop ADCs with high clinical efficacy, these toxic agents are usually modified to improve their virulence in cancer cell killing and to make them susceptible for linker mediated antibody conjugations. Several modifications of bleomycin A2 have been made for this very purpose: for instance, variations in the L-threonine subunit substituents have been prepared and they showed a strong impact on the cleavage efficiency of the modified bleomycin A2. However, this modification has no obvious effects on the cleavage selectivity.
With our well-established "DrugLnk" organic synthesis platform and rich experience in payload chemistry, scientists here at Creative Biolabs are dedicated to help our clients develop modified bleomycin A2 and bleomycin A2-linker complexes using readily available or customized linkers for antibody conjugation in a timely and cost-effective manner. Our customarily tailored services and high quality products will contribute greatly to the success of your projects.
Creative Biolabs also provides other various services regarding ADC development. Please feel free to contact us for more information and a detailed quote.
References:
- Viraja R, Palwai, and Eriksson Leif A. "Molecular dynamics simulations exploring the interaction between DNA and metalated bleomycin." Journal of Biophysical Chemistry 2011 (2011).
- Murray, Vincent, Jon K. Chen, and Long H. Chung. "The interaction of the metallo-glycopeptide anti-tumour drug bleomycin with DNA." International journal of molecular sciences 19.5 (2018): 1372.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
