- Home
- ADC Development
- DrugLnk™ Custom Linker-Payload Synthesis
- Drug Module Synthesis
- DNA Toxin Synthesis
- Dactinomycin Synthesis
Dactinomycin Synthesis Service
As a leader in antibody engineering and bio-conjugation chemistry, Creative Biolabs has established an advanced "DrugLnk" platform for customized antibody-drug conjugates (ADCs) development. We offer comprehensive services for the preparation of ADCs and other targeted antibody therapeutics bearing dactinomycin D and its analogues as payloads.
Dactinomycins, also known as actinomycins, are antibiotic metabolites derived from several species of Streptomyces. Dactinomycin D, a potent derivative in the dactinomycin superfamily, shows both strong antibacterial and antitumor activity. It has been extensively exploited in clinical practices as an anticancer drug since 1954. Dactinomycin D suppresses DNA replication and it has been utilized for the treatment of neuroblastoma and lymphomas. Dactinomycin D is comprised of a central phenoxazone ring containing two peptides containing D-amino acids.
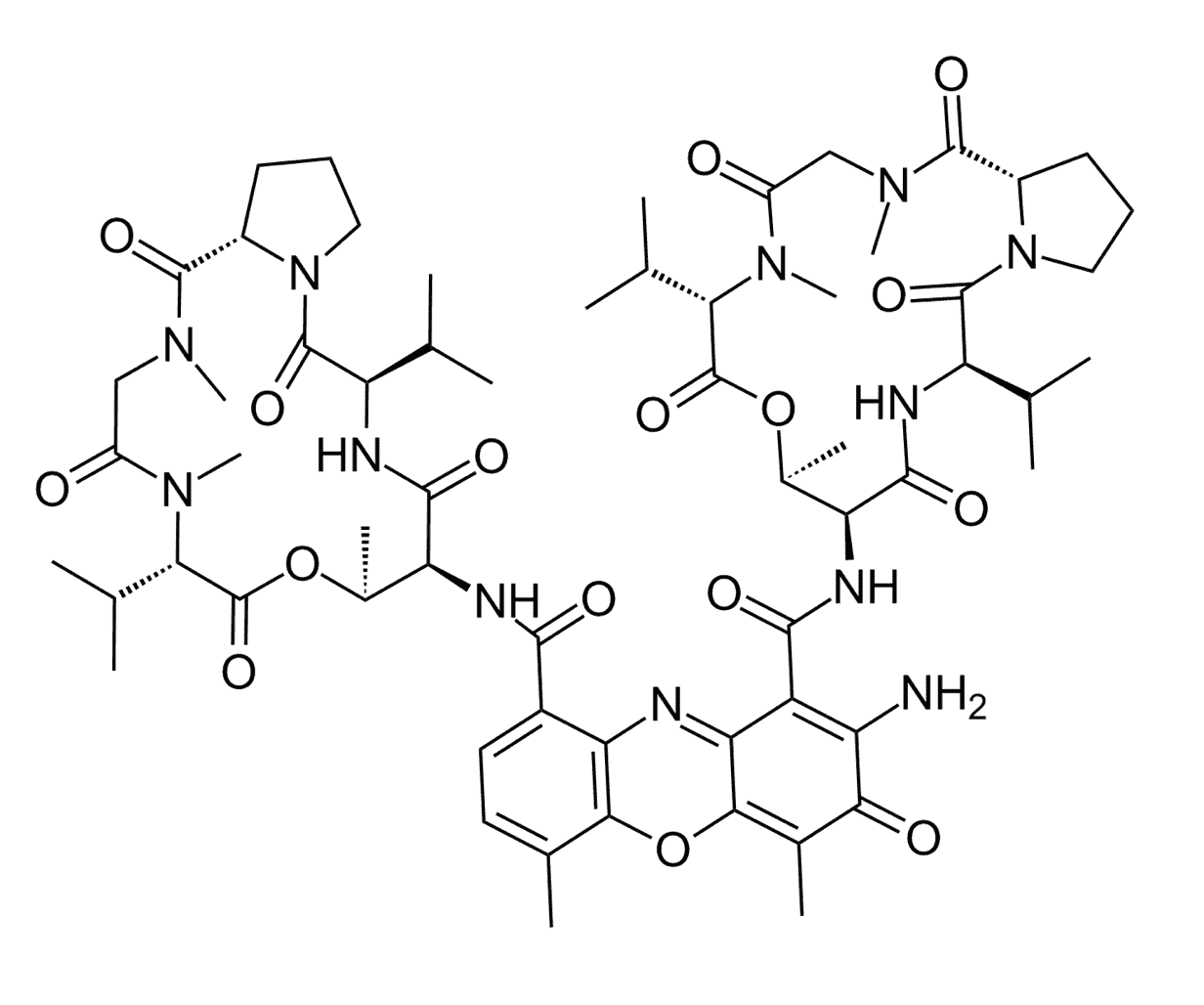 Fig.1 The chemical structure of dactinomycin D.1,2
Fig.1 The chemical structure of dactinomycin D.1,2
Earlier studies have shown that dactinomycin D is able to suppress the acute bronchoconstriction effects of tachykinin neurokinin A (NKA), an extremely strong regulatory action in the mitogenic response caused by phytohemagglutinin (PHSA) in human peripheral blood mononuclear cells. It is also speculated that the mechanism for dactinomycin D transmembrane transportation is related to the interaction with tachykinin receptors.
Dactinomycin D Mode of Action (MOA) in Cancer Treatments
The cytotoxic and antitumor actions of dactinomycin D is associated with the disruption of DNA transcription machinery that leads to RNA and protein biosynthesis. Two proposed MOA of dactinomycin D include the prevention of RNA polymerases progression via DNA intercalation and the stabilization of the molecular complexes formed by DNA and topoisomerases I and II. In the later MOA, the phenoxazone ring of dactinomycin D is inserted between GpC base pair sequences in the double-stranded DNA. In the meantime, the polypeptide lactones rings of dactinomycin D occupy a position in the minor groove of the DNA helix where the association of topoisomerase with DNA occurs. The association of dactinomycin D with both DNA and topoisomerase results in a stabilized DNA-topoisomerase complex that halts transcription. What's more, several other crucial factors also enhance the anti-tumor activity of dactinomycin D, including its slow dissociation from DNA complexes, its photodynamic activity, and free radical formation.
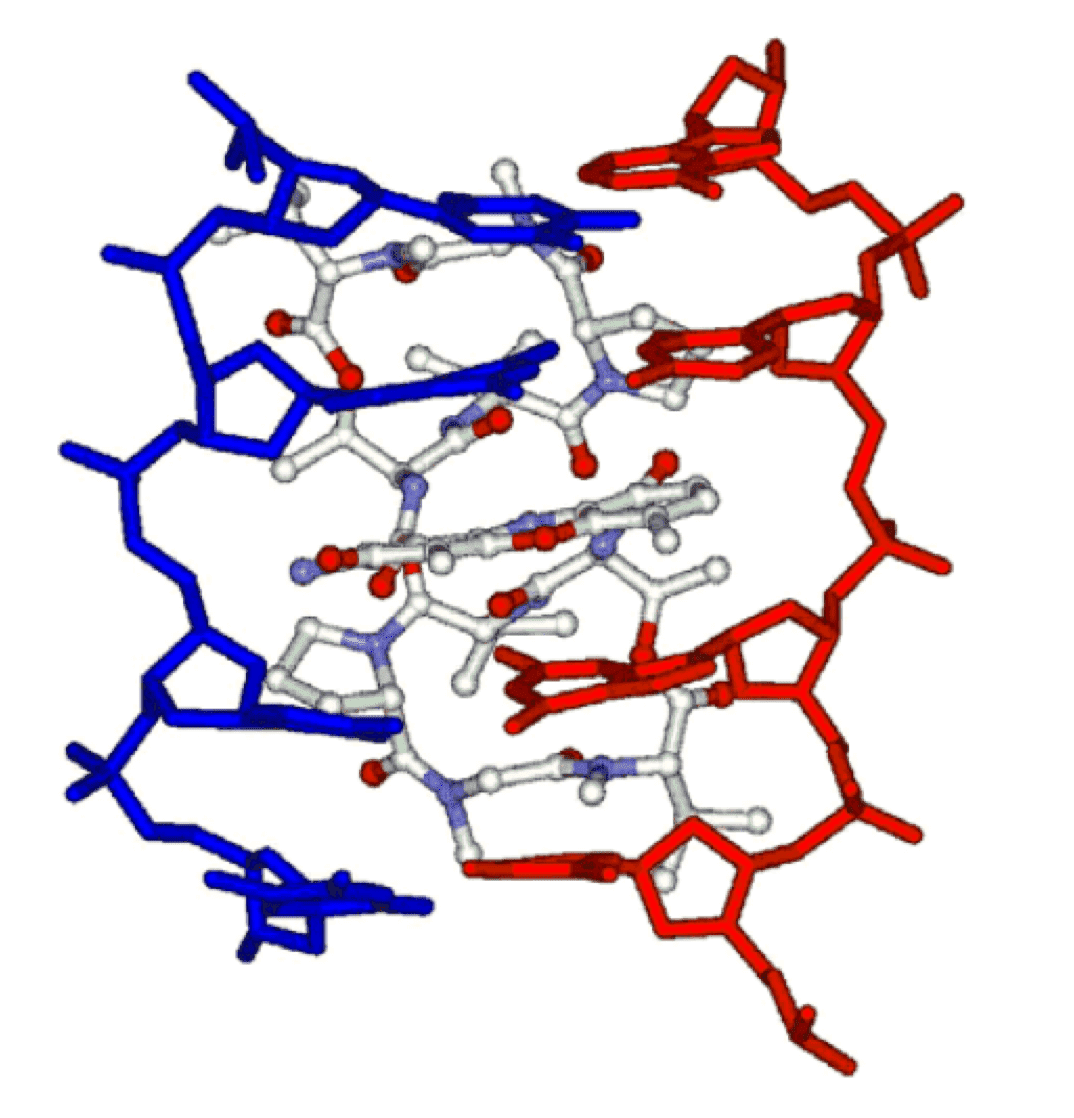 Fig.2 Schematic representation of the dactinomycin D-TGCA sequence complex interface.1,2
Fig.2 Schematic representation of the dactinomycin D-TGCA sequence complex interface.1,2
Dactinomycin D-based ADCs
ADCs are a novel class of biopharmaceutics aiming at the accomplishment of site-specific delivery of cytotoxic agents to tumor cells. The exploitation of ADCs is a promising strategy to overcome the disadvantages of traditional pharmacotherapies of cancer. Several previous studies have accomplished the modification of dactinomycin D to form prototypes that are more susceptible for bio-conjugation, producing a number of dactinomycin D analogs. For example, dactinomycin D actinocin derivatives, comprised of benzo-15-crown-5 and benzo-18-crown-6 radicals, enable either direct conjugation to phenoxazinee chromophore or an indirect conjugation via spacers of various lengths to antibodies.
With our well-established "DrugLnk" organic synthesis platform, the experienced scientists here at Creative Biolabs are dedicated to help you develop dactinomycin D-linker complexes using readily available or customized linkers for antibody conjugation in a timely and cost-effective manner. Our customarily tailored services and high quality products will contribute to the success of your projects.
Creative Biolabs also provides other various services regarding ADC development. Please feel free to contact us for more information and a detailed quote.
References:
- Wang, Sheng-Yu, et al. "Spermine attenuates the action of the DNA intercalator, actinomycin D, on DNA binding and the inhibition of transcription and DNA replication." PloS one 7.11 (2012): e47101.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
