HIV infects cells of the host immune system, which can be targeted and edited through gene therapy. A gene-editing platform based on the clustered regularly spaced palindromic repeat-cas system (CRISPR-Cas) has been recognized as a promising tool for developing gene therapies for HIV infection. It is a promised approach that antibody gene transfer immunoprophylaxis, which involves viral delivery of genes that encode broadly neutralizing antibodies (bNAbs). Besides, bNAb gene delivery to stem and progenitor cells in humanized mice has been described.
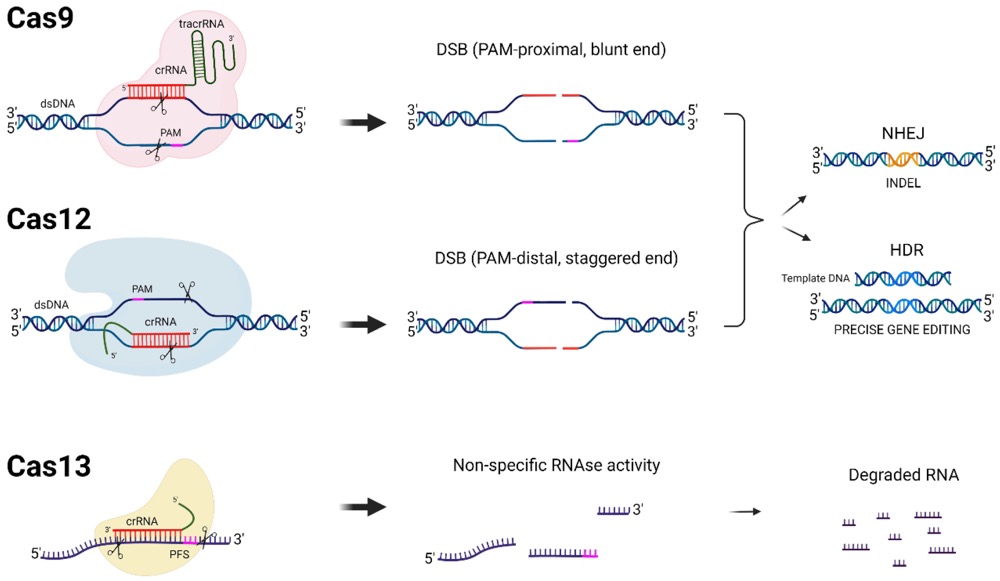 Fig.1 DNA- and RNA-targeting CRISPR-Cas systems. (Hussein, et al., 2023)
Fig.1 DNA- and RNA-targeting CRISPR-Cas systems. (Hussein, et al., 2023)
Creative Biolabs engineers primary B cells with precise genome editing techniques, which replace endogenously encoded antibodies of primary human B cells with antibodies that target HIV. Engineered B cells enable immune memory and clonal selection, which may help address viral variability and counteract viral escape.
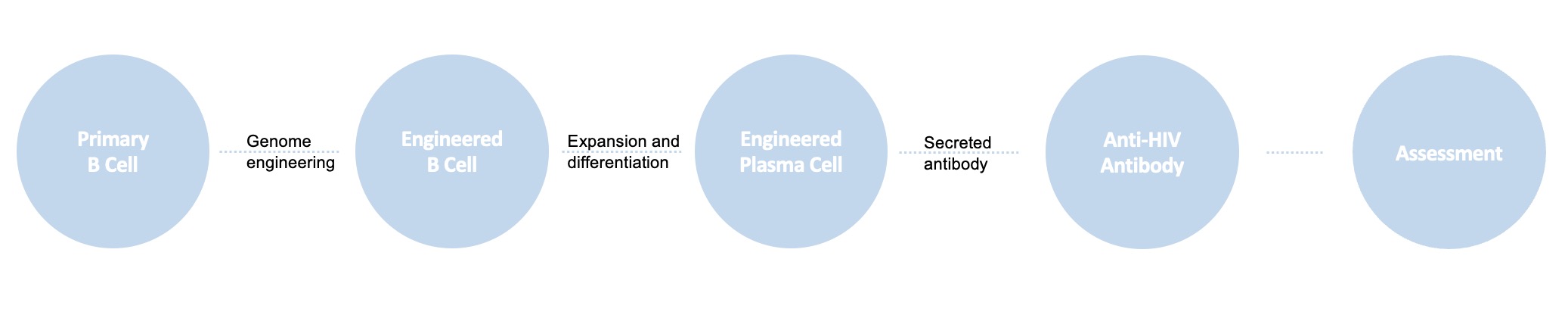 Fig.2 B cell engineering service for anti-HIV antibody delivery. (Creative Biolabs)
Fig.2 B cell engineering service for anti-HIV antibody delivery. (Creative Biolabs)
Paper Title: Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion
Technology: CRISPR/Cas9-based Genome Editing
Therapeutic Antibody: Anti-HIV Antibody
Journal: Cell
IF: 14.919
Published: 2020
Background: B cell engineering therapy can overcome the challenges, such as bNAbs therapy which chronically administered at a higher cost. This research engineered B cells by CRISPR/Cas9 to express anti-HIV antibodies which are capable of secreting high antibody titers. This shows that B cells can be engineered into what could be a living and evolving drug for HIV.
Results: The engineered B cells enable immunological memory and clonal selection that may contribute to addressing viral variability between patients and to counteracting viral escape.
Following are some results displayed that refer to B cell engineering for Anti-HIV antibody delivery in this article:
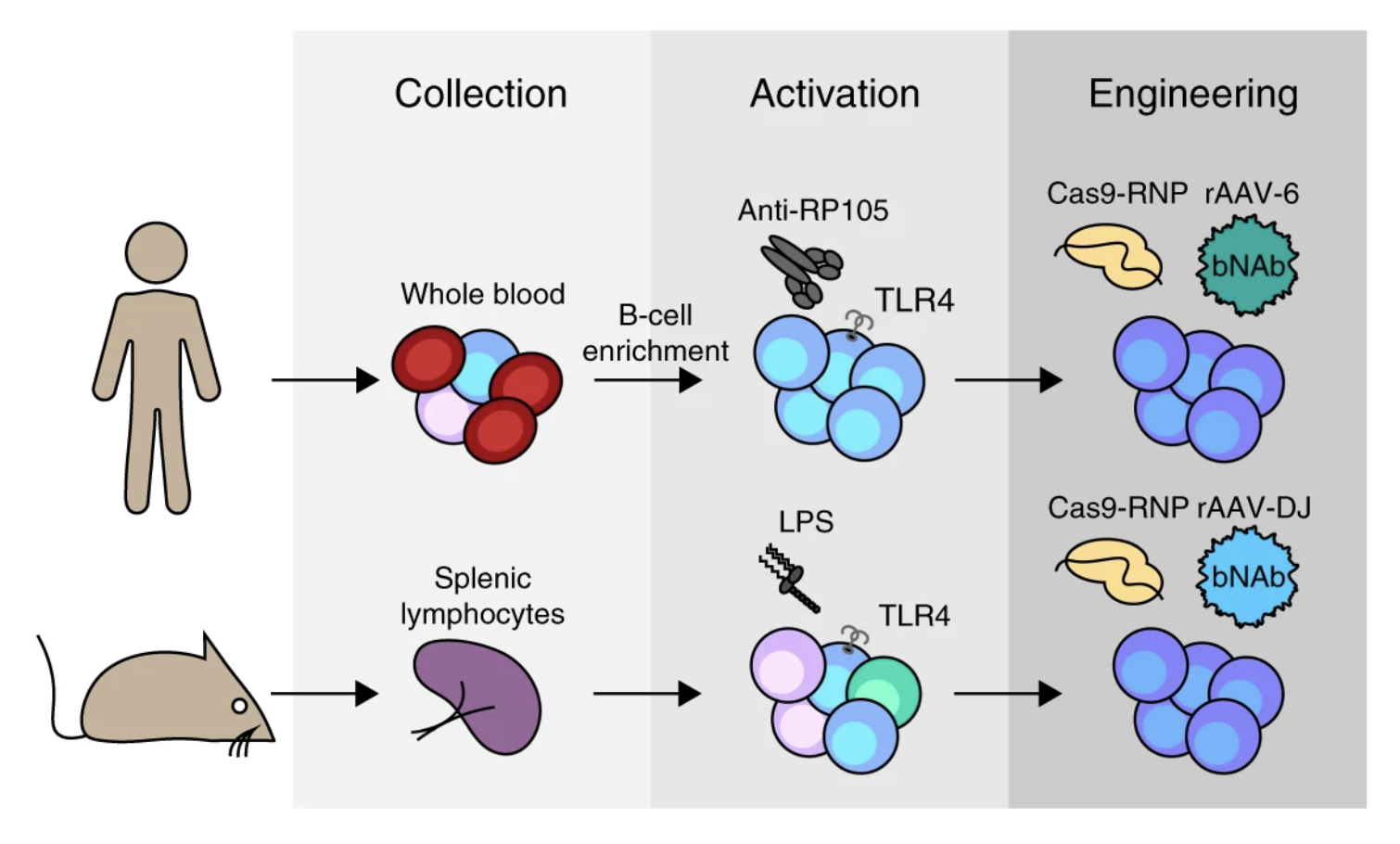 Fig.3 The protocol of the human and mouse B cell engineering. (Nahmad, et al., 2020)
Fig.3 The protocol of the human and mouse B cell engineering. (Nahmad, et al., 2020)
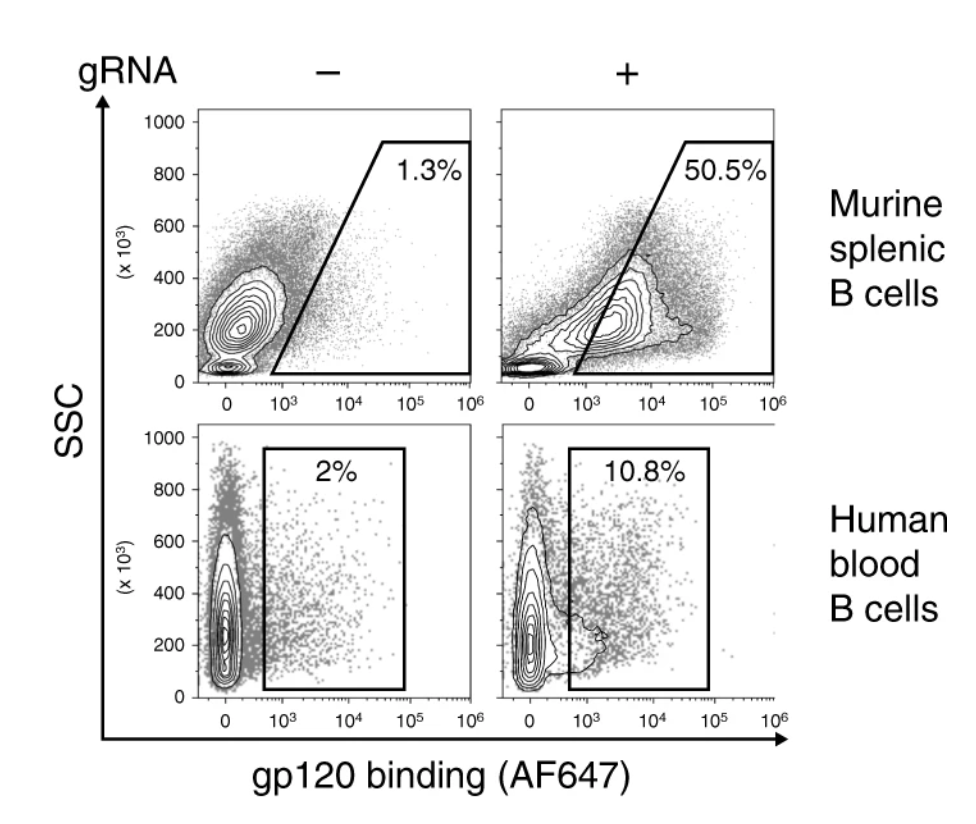 Fig.4 Flow cytometry of binding of the HIV gp120 antigen by the 3BNC117 BCR. (Nahmad, et al., 2020)
Fig.4 Flow cytometry of binding of the HIV gp120 antigen by the 3BNC117 BCR. (Nahmad, et al., 2020)
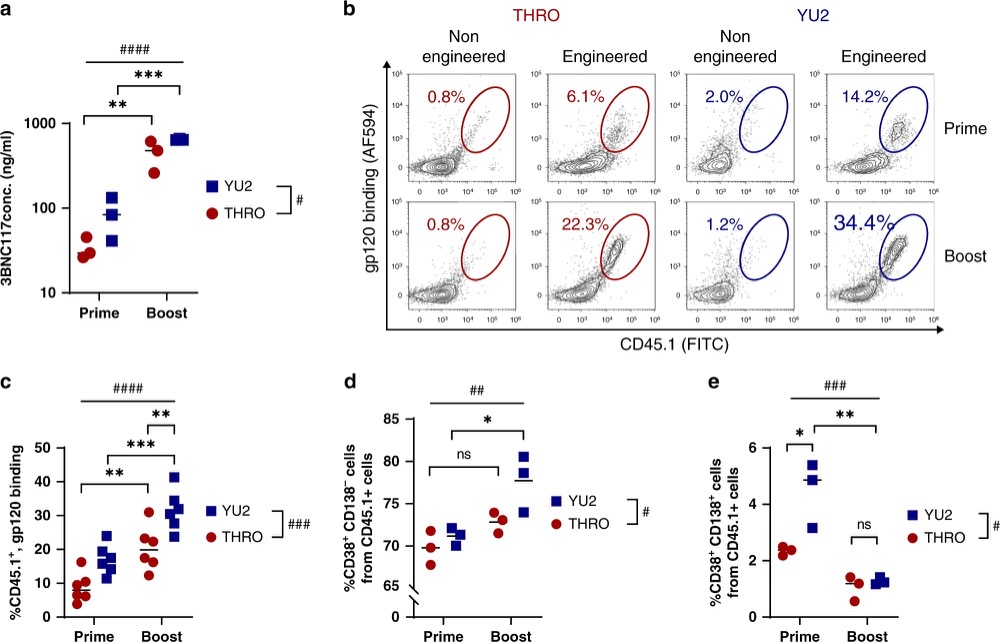 Fig.5 Adoptively transferred engineered B cells enable memory retention upon immunization. (Nahmad, et al., 2020)
Fig.5 Adoptively transferred engineered B cells enable memory retention upon immunization. (Nahmad, et al., 2020)
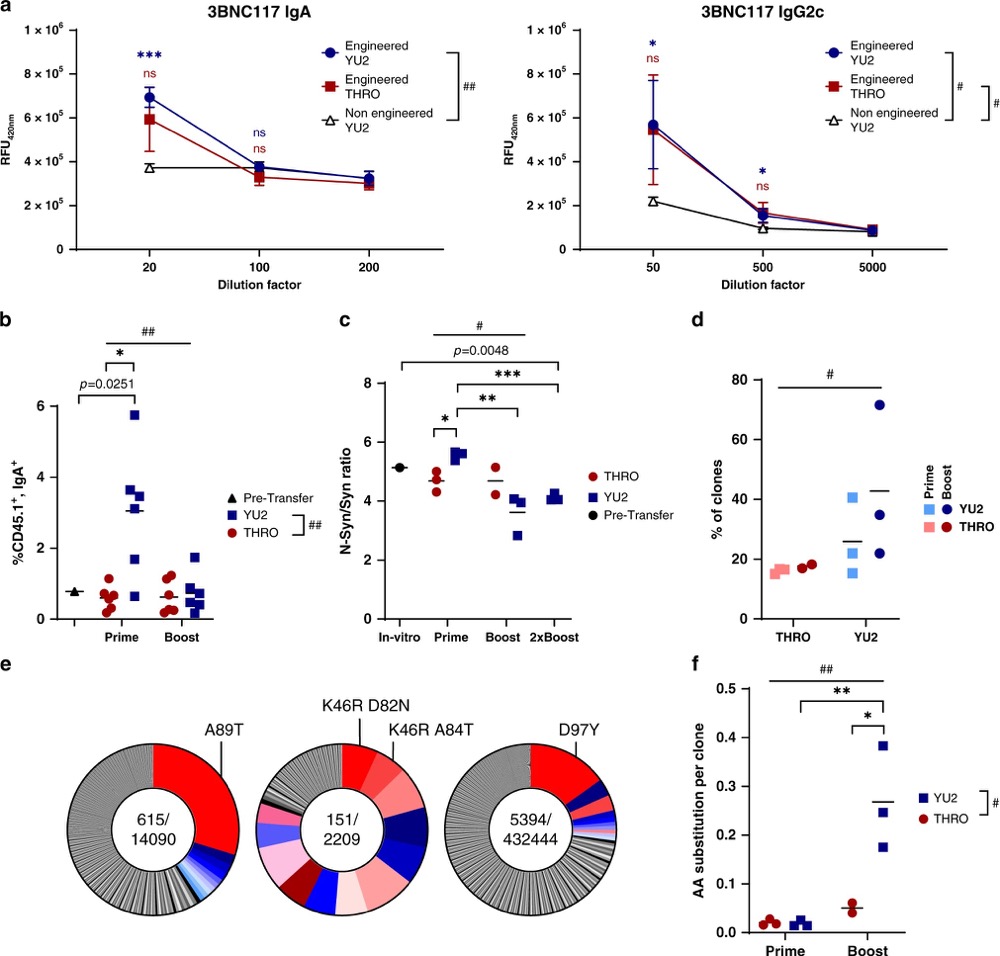 Fig.6 Adoptively transferred engineered B cells can clonal expansion upon immunization and undergo CSR. Nahmad, et al., 2020)
Fig.6 Adoptively transferred engineered B cells can clonal expansion upon immunization and undergo CSR. Nahmad, et al., 2020)
Q1: Why choose B cell engineering for anti-HIV antibody delivery?
A: The engineered B cell will overcome the following challenges.
Q2: What is the approach of CRISPR/Cas anti-HIV?
A: CRISPR/Cas anti-HIV approaches used to protect cells from HIV infection are shown as follows.
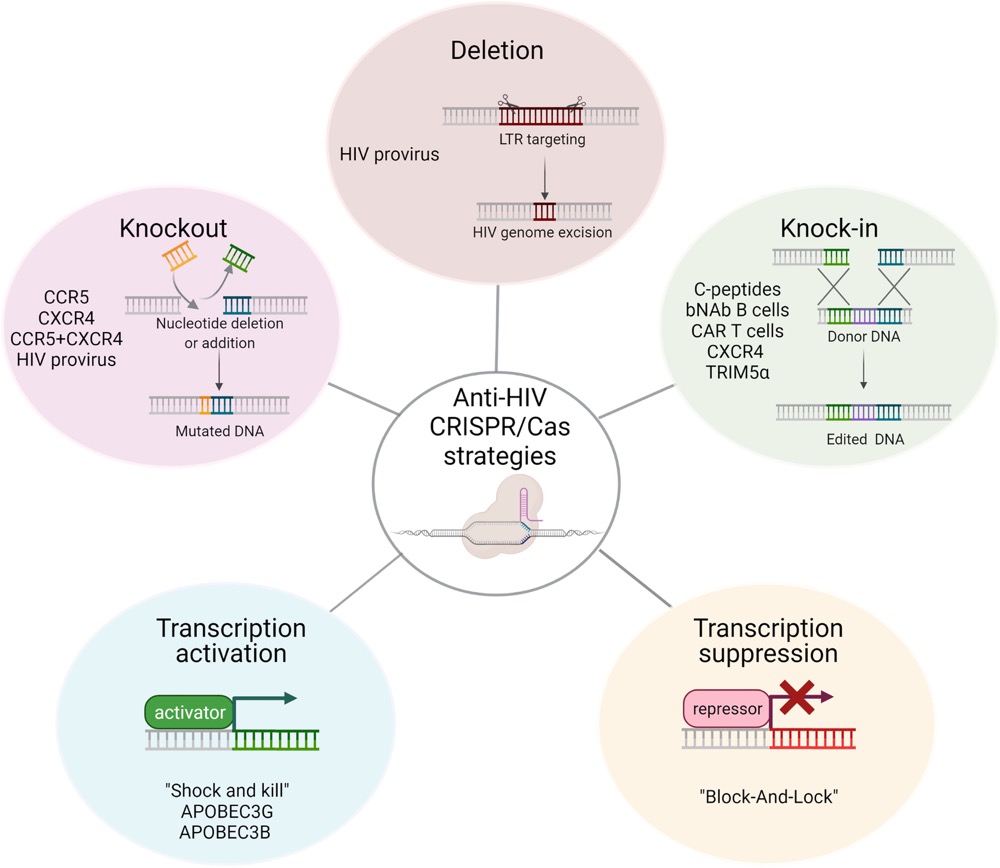 Fig.7 CRISPR/Cas anti-HIV approaches. (Maslennikova, et al., 2022)
Fig.7 CRISPR/Cas anti-HIV approaches. (Maslennikova, et al., 2022)
Q3: What other diseases can B cell engineering technology be used for?
A: As a platform technology, B cell engineering can be applied to diverse persistent infections as well as to the treatment of congenital disorders, autoimmune diseases and cancer. For more information on this, please browse below:
|
|
B Cell Engineering Service for Anti-COVID-19 Antibody Delivery |
| Creative Biolabs provides the B cell engineering service to manufacture antibodies to defend against the invasion of COVID-19 virus. Please click to know more. | |
|
|
B Cell Engineering Service for Anti-RSV Antibody Delivery |
| Creative Biolabs provides B cell engineering services by different genome editing methods to manufacture antibodies to defend against the invasion of RSV. Please click to know more. | |
|
|
B Cell Engineering Service for Anti-Influenza Virus Antibody Delivery |
| Creative Biolabs provides the B cell engineering service to manufacture antibodies to defend against the invasion of the influenza virus. Please click to know more. | |
|
|
B Cell Engineering Service for Anti-EBV Antibody Delivery |
| Creative Biolabs provides B cell engineering services to manufacture antibody by to defend the invasion of EVB. Please click to know more. |
We offer professional consultation guidance before, during and after the project, please contact us for your tailored solution.