Creative Biolabs is an undisputed leading provider of antibody development and generation services. Now, we provide in vitro diagnostic (IVD) antibody development services targeting various biomarkers of a wide variety of diseases. Especially, Creative Biolabs offers high-quality antibody development services for diagnostic applications targeting the fatty acid binding protein (FABP) marker.
Fatty Acid Binding Protein (FABP)
The fatty-acid-binding proteins (FABPs) are a family of small cytoplasmic transport proteins expressed in the tissues with an active fatty acid metabolism. The main function of FABPs is to facilitate the transfer of long-chain fatty acids between extra- and intracellular membranes.
Heart-type fatty acid binding protein (hFABP), also known as mammary-derived growth inhibitor, is a protein that in humans is encoded by the FABP3 gene. H-FABP is a 15 kDa cytoplasmic protein released from cardiac myocytes following an ischemic episode. H-FABP is involved in active fatty acid metabolism where it transports fatty acids from the cell membrane to mitochondria for oxidation. The FABPs belongs to a multigene family, which has at least three distinct types, the hepatic-, intestinal- and cardiac-type. They are thought to participate in the uptake, intracellular metabolism and/or transport of long-chain fatty acids. They may also be responsible in the modulation of cell growth and proliferation.
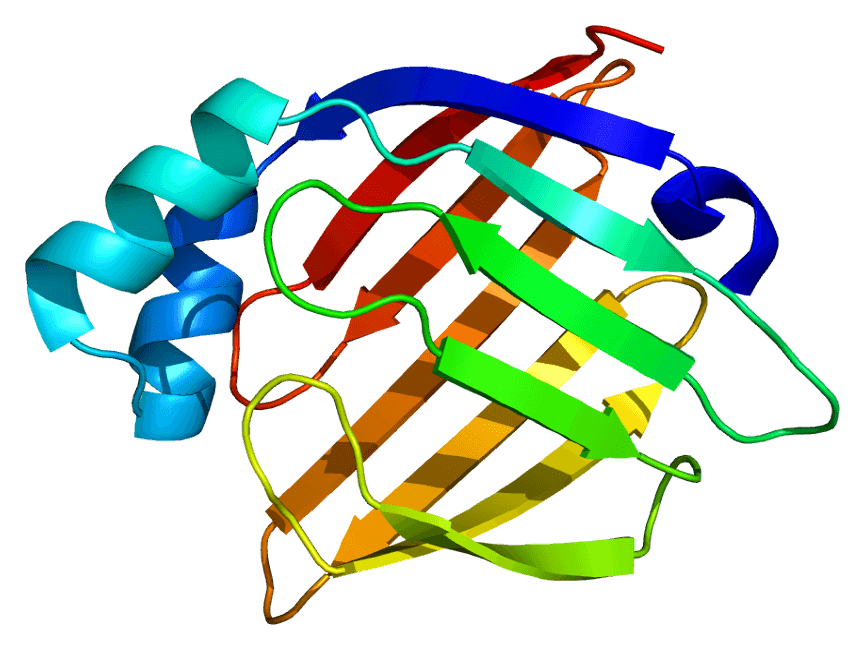 Fig.1 Structure of the H-FABP protein.Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Fig.1 Structure of the H-FABP protein.Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Liver-type fatty acid-binding protein (L-FABP), frequently known as fatty acid-binding protein 1, is encoded by the FABP1 gene in human. L-FABP is predominantly expressed in the liver where it participates in the binding, transport and metabolism of long-chain fatty acids (LCFAs), phytocannabinoids, endocannabinoids (and less so for synthetic cannabinoid receptor (CBR) agonists and antagonists), as well as other hydrophobic molecules. L-FABP has been reported to have an exclusive structure in comparison with other members of the FABP family, which enables it to bind multiple ligands simultaneously, including bilirubin, bile acids, monoglycerides and fatty acyl CoA. It also has a larger solvent-accessible core that allowing a greater variety of substrate binding. It has been proposed that fatty acids enter the solvent-accessible area of L-FABP by a dynamic region composed of α-helix II and turns between βC-βD and βE-βF loops. Subsequently, the fatty acid is bound in the cavity of L-FABP for transport.
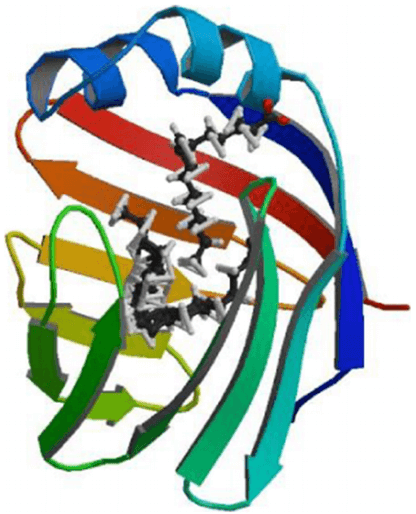 Fig.2 L-FABP structure.1
Fig.2 L-FABP structure.1
H-FABP Marker of Neurological Disease
In adult brain, H-FABP is predominantly found in neuronal tissue (neuronal cell body) and constitutes 0.01% of the total brain cytosolic protein. The distinction between dementia with Lewy bodies (DLB) and Alzheimer's disease (AD) remains challenging due to the significant overlap in clinical signs. Today a definite diagnosis of the underlying neurodegenerative syndrome still requires neuropathological confirmation. H-FABP together with other protein has the potential to be of use in the differential diagnosis of several neurodegenerative diseases, especially the distinction between DLB and AD.
Recently, HFABP is identified as a potential CSF biomarker for Creutzfeldt-Jakob disease (CJD). H-FABP has been found to display a wide tissue distribution including the expression in brain. FABP is alpha-synuclein, which is genetically and neuropathologically linked to Parkinson disease (PD) and DLB and was recently shown to function as a FABP-like constituent of neurons and to affect lipid metabolism in vivo. The measurement of biomarkers in cerebrospinal fluid (CSF) has gained increasing acceptance in establishing the diagnosis of some neurodegenerative diseases. H-FABP has recently been discovered in CSF and serum of patients with neurodegenerative diseases.
H-FABP Marker of Myocardial Infarction
MI, also known as heart attack, is a type of acute coronary syndrome. It is the leading cause of mortality and disability all over the world. MI occurs when a coronary artery is sudden blockaded with subsequent myocardial ischaemia, causing damage to the surrounding heart muscle. Determination of quickly arising cardiac biomarker in plasma enables to offer more suitable diagnosis for patients with MI, especially when signals and electrocardiograms are suspicious. It has been demonstrated that H-FABP is a potential marker of cardiac injury in the early asymptomatic period in patients with MI. The concentration of FABP in cardiac tissue is 20 times higher than that in skeletal muscle, which makes H-FABP more cardiac specific than myoglobin. Besides, H-FABP is able to be released from severely injured cardiomyocytes to the blood more quickly than other markers, including cTn-I, CK-MB and MYO. What's more, as a marker of MI, H-FABP even can be used in the absence of frank necrosis, and may be a helpful tool as prognostic value for detecting of a recurrent infarction.
 Fig.3 Hypertrophic decidual vasculopathy. Distributed under CC BY 3.0, from Wiki,
without modification.
Fig.3 Hypertrophic decidual vasculopathy. Distributed under CC BY 3.0, from Wiki,
without modification.
H-FABP Marker of Heart Failure
HF, also known as congestive heart failure, happens when the heart muscle can't pump enough blood it required. Several situations may slowly make the heart too weak or stiff to fill and pump available, for example, high blood pressure or narrowed arteries in your heart. Recently, a study revealed that additional H-FABP detection enhanced the diagnostic specificity of single NT-proBNP testing in patients with HF, suggesting that H-FABP is a promising marker for the diagnosis of HF.
L-FABP Marker of Renal Hypoxia
FABPs (Fatty acid–binding proteins) have been shown to bind unsaturated fatty acids and lipid peroxidation products during tissue injury from hypoxia. L-FABP is expressed primarily in the proximal tubules of human kidney, a nephron segment that uses fatty acids as the main source of energy metabolism, and shed into urine in response to hypoxia resulted from decreased peritubular capillary blood flow. And it was thought to participate in energy production/metabolism in renal tubule cells. Urinary L-FABP (L-type FABP) level is found to have a significant direct correlation with both peritubular capillary blood flow and the ischemic time of the transplanted kidney. In studies on human L-FABP transgenic mouse models, the results demonstrate that the expression of renal L-FABP protects kidney tissue from renal ischemic stress. It has also been suggested that L-FABP plays a crucial role in preventing cytotoxicity by binding fatty acids, heme and other molecules that have potential toxicity when unbound.
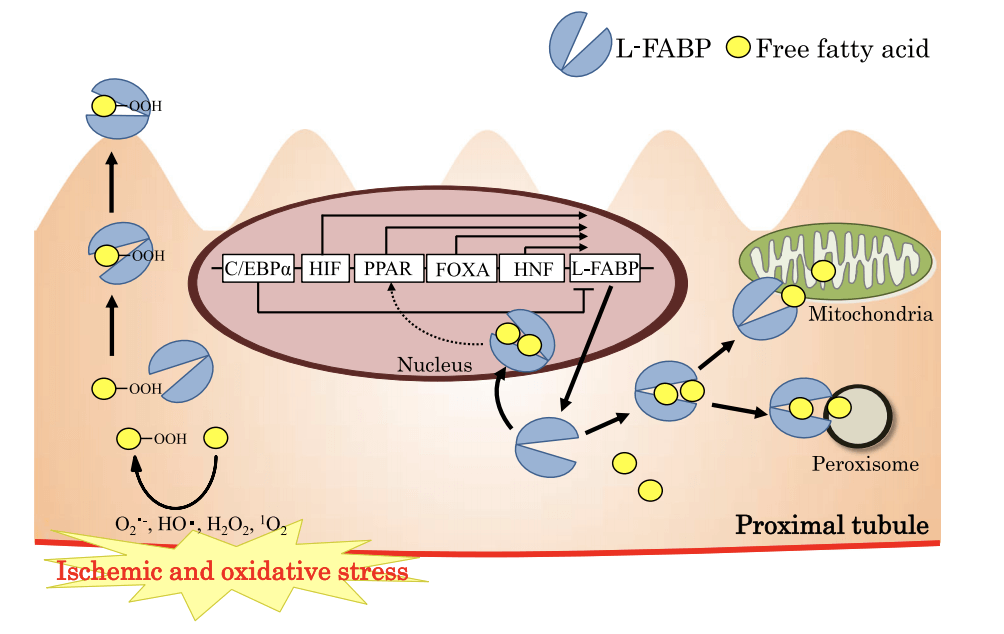 Fig.4 L-FABP urinary excretion model.1
Fig.4 L-FABP urinary excretion model.1
IVD Antibody Development Services Targeting FABP Marker
Antibodies are core elements for antibody-based immunoassays for detecting and quantifying antigens of interest in all kinds of samples such as the serum, urine, tissue preparations, and so on. IVD antibodies are extensively used for disease screening, prognosis, and therapeutic monitoring. With our versatile IVD platform, Creative Biolabs is proud to develop novel anti-FABP antibodies from scratch to commercial IVD kit (we can also start with provided antibody candidates).
If you are interested in our IVD antibodies discovery services, please contact us for more details.
Reference
- Yokoyama, Takeshi, et al. "Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage." The American journal of pathology 174.6 (2009): 2096-2106. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.