In vitro diagnostics (IVD) has moved from morphology based cellular analysis to incorporating molecular characterization. Biomarker analysis at the protein level provides a powerful approach to measuring biological process indicators in normal and pathological conditions or response to pharmacological intervention. Established techniques such as Microfluidics, Liquid Biopsy, Array Technique and perfect Diagnostic Platform at Creative Biolabs offer trustworthy systems on which to build and offer high-quality protein biomarker services for global clients.
Protein Detection Methods
A variety of protein assays for disease diagnosis, including those based on enzyme linked-immunosorbent assay (ELISA), immunohistochemistry (IHC), and flow cytometry (FC), are currently available in research use. At Creative Biolabs, work continues to be done to improve sensitivity and specificity as well as to improve the multiplexing and analysis capabilities for these methods. Some emerging protein analysis techniques such as mass spectrometry (MS), proximity assays are used extensively for research purposes.
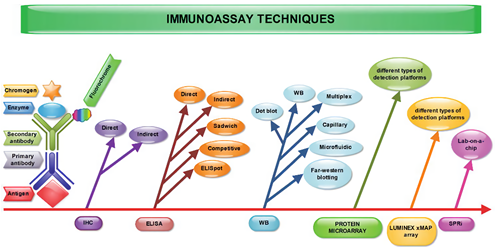 Fig.1 The schematic representation of the immunoassay methods. (Manole, 2018)
Fig.1 The schematic representation of the immunoassay methods. (Manole, 2018)
One emphasis in IVD is the move to methods that use increasingly smaller sample sizes and more portable equipment. Miniaturization reduces cost and allows for easier multiplexing. Microfluidics reduces assay variability by controlling fluid flow and reduces labor through the possibility of automation. Micro and nanoparticles improve consistency between reactions and concentrate products. Colorimetric, fluorescent and electrochemical, chemiluminescence, electrochemiluminescence and other detection mechanisms have been extensively applied to disease biomarker detection.
Cases in Disease Diagnosis
To date, published examples of protein microarrays for IVD purposes have included tests for autoimmune, cancer, infectious, and cardiovascular diseases. In the context of infectious diseases, different types of protein-based laboratory tests can identify microorganisms such as bacteria, viruses, fungi, and parasites. There are two major types of diagnostic technologies: antibody test and antigen test. For instance, studies on the serum of recovered COVID-19 patients suggest that host-neutralizing antibodies primarily work against spike (S) and nucleocapsid (N) proteins. The likelihood of predicting immunity status could increase in serological tests that target various regions of S or N proteins. Among four major types of serological diagnostic tests, the rapid diagnostic test (RDT) is a simple and rapid test based on lateral flow immunoassay (LFIA) technology and can potentially be administered as a point-of-care (POC) test or self-test. As Fig.2 shown, RDT test strips use a drop of blood to detect the presence of patient antibodies (IgG, IgM, or IgA) produced against a specific SARS-CoV-2 antigen.
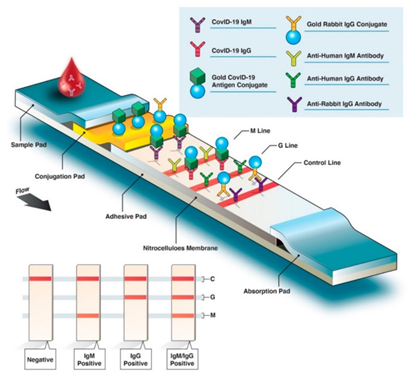 Fig.2 Overview of COVID-19 rapid diagnostic test for IgG and IgM detection.2, 4
Fig.2 Overview of COVID-19 rapid diagnostic test for IgG and IgM detection.2, 4
Besides, the analytical protein array has been applied to identify biomarkers for a wide variety of cancers since it allows simultaneously monitoring hundreds of thousands of protein samples in cells or tissues. Taking breast cancer as an example, analytical protein arrays have been widely adopted as an unbiased screening tool for profiling the protein contents in cell lines, tissues, and patient sera. Some potential biomarkers, including p53 (TP53), annexin A11 (ANX11), mitogen-activated protein kinase 7 (MAPK7), cell division cycle 25C (CDC25C), and eukaryotic translation initiation factor 4E (EIF4E) were successfully identified to have increased expression levels in breast cancer.
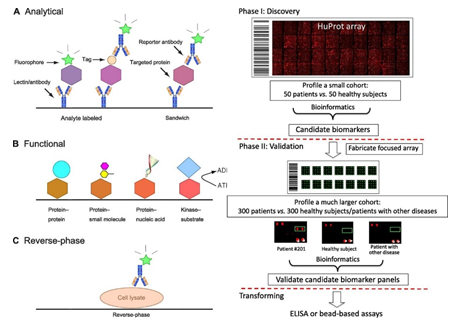 Fig.3 Types of protein arrays (left) and scheme of the two-phase strategy for biomarker identification using functional protein arrays (right).3, 4
Fig.3 Types of protein arrays (left) and scheme of the two-phase strategy for biomarker identification using functional protein arrays (right).3, 4
What Creative Biolabs Can do for You?
Our scientists have extensive experience setting up biomarker assays to quantitate proteins in various biological matrices such as blood, plasma, serum, urine, cerebrospinal fluid (CSF), organ tissue homogenate, etc. These biomarker studies can be performed using a traditional ELISA assay or one of our advanced biomarker assay platforms (LC-MS/MS, MSD ECL). We offer these biomarker testing services for the analysis of your study samples through custom assay development or commercially available assay kits.
- Improved biorecognition molecules such as antibodies and aptamers to increase detection specificity and sensitivity
- Integration of Microfluidics with biorecognition molecules to improve affordability by miniaturizing the system and repeatability.
- New materials and electronics will be used to reduce background, decrease cost, and improve sensitivity.
- Combinational detection of nuclear acid and proteins.
For more information, please contact us.
Published Data
1. Serological Biomarker Detection for Lung Cancer Early Diagnosis Using Protein Array Technology
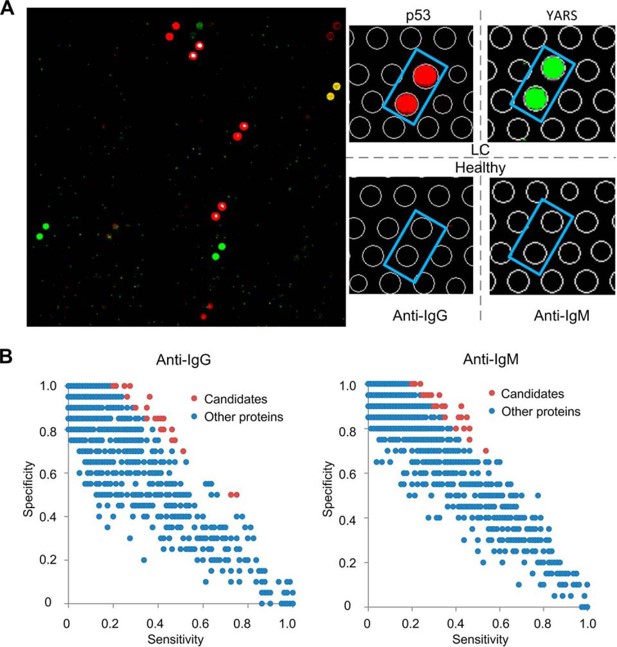 Fig.4 Serum profiling assays on protein arrays in Phase I.5,4
Fig.4 Serum profiling assays on protein arrays in Phase I.5,4
To address the lack of serological biomarkers for early lung cancer (LC) diagnosis, this study employed a two-phase strategy through a protein array-based method. In Phase I, serological autoimmune profiles from 80 LC patients and 20 healthy individuals were analyzed, identifying 170 candidate proteins strongly linked to LC. In Phase II, a lung cancer-focused array was created using these 170 biomarkers and used to profile a large cohort comprising 93 healthy individuals, 352 LC patients, and 101 patients with lung benign lesions (LBL). By comparing autoimmune profiles between early-stage LC and the combined group of healthy and LBL individuals, a biomarker panel consisting of HRas, p53, and ETHE1 was identified, demonstrating 50% sensitivity and over 90% specificity for diagnosing early-stage LC. The panel's performance was further validated through ELISA tests. This study represents a comprehensive proteome-wide survey with the largest and most diverse cohorts.
References
- Manole, Emilia, et al. "Immunoassay techniques highlighting biomarkers in immunogenetic diseases." Immunogenetics. IntechOpen, 2018. Distributed under Open Access license CC BY 3.0, without modification.
- Ghaffari, Abdi, Robyn Meurant, and Ali Ardakani. "COVID-19 serological tests: how well do they actually perform?." Diagnostics 10.7 (2020): 453.
- Huang, Yi, and Heng Zhu. "Protein array-based approaches for biomarker discovery in cancer." Genomics, Proteomics and Bioinformatics 15.2 (2017): 73-81.
- Distributed under Open Access license CC BY 4.0, without modification.
- Pan, Jianbo, et al. "Identification of serological biomarkers for early diagnosis of lung cancer using a protein array-based approach." Molecular & Cellular Proteomics 16.12 (2017): 2069-2078.
For Research Use Only.