Renal Cell Carcinoma Tissue Exosome Research & Application
Introduction Research Direction Services FAQs
A More Authentic Representation of the Communication Network Driving Renal Cell Carcinoma Progression
Renal cell carcinoma (RCC) is the most prevalent form of kidney malignancy, yet its early detection remains a significant challenge. A majority of patients are diagnosed at advanced stages, when the disease has already metastasized, highlighting the critical need for novel, effective biomarkers and therapeutic targets. For years, the scientific community has been captivated by the potential of exosomes—nanoscale membranous vesicles that facilitate intercellular communication by transporting a cargo of proteins, nucleic acids, and lipids. These vesicles are secreted into the tissue microenvironment and can circulate throughout the body, making them promising candidates for diagnostic and therapeutic applications.
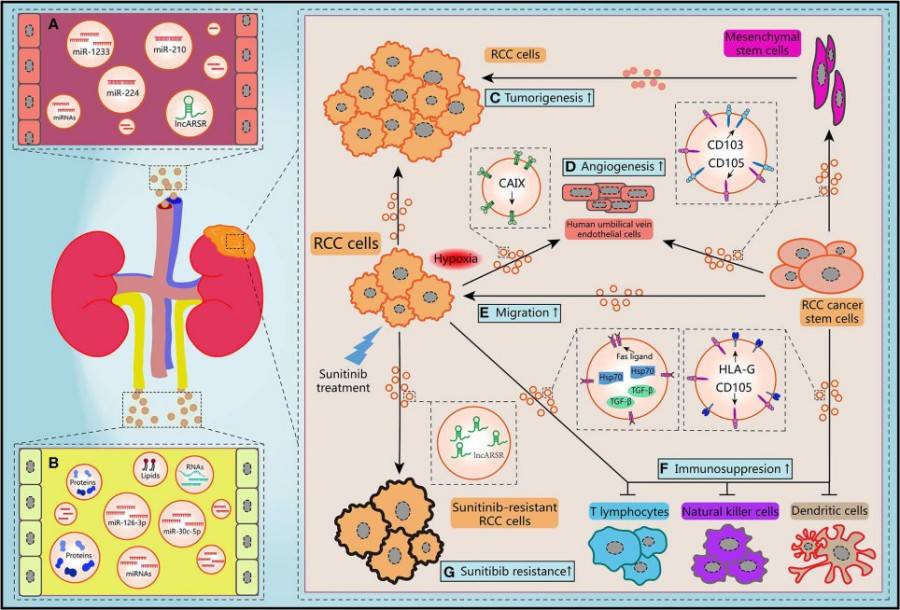 Fig.1 Schematic diagram of the biological features of EVs.1
Fig.1 Schematic diagram of the biological features of EVs.1
Early research into RCC exosomes primarily focused on those isolated from cell culture supernatants or systemic biofluids like blood and urine. While these studies have provided valuable insights into tumor proliferation, metastasis, angiogenesis, and immune evasion, they are limited by a fundamental issue: authenticity.
 Cell Culture Exosomes
Cell Culture Exosomes
Derived from an individual cell type in a controlled lab setting, they fail to replicate the complexity of the in vivo tumor microenvironment.
 Systemic Biofluid Exosomes
Systemic Biofluid Exosomes
A heterogeneous mix originating from various tissues and organs, which can obscure the specific signals unique to the kidney tumor.
Creative Biolabs offers a range of tissue exosome functional research and verification services. These services include in vivo functional research and disease model construction services for cancer and other relevant diseases, enabling researchers to study the biological activity and therapeutic potential of exosomes in vivo. By providing a direct window into the disease at its source, tissue-derived exosomes offer a more authentic representation of the communication network driving RCC progression, unlocking new opportunities for the discovery of highly specific biomarkers and the development of targeted therapies.
Unlock Your Research Potential. Request a consultation to discuss your project.
Research of the Role of RCC Tissue Exosomes
We have closely followed key findings that underscore the immense potential of tissue-derived exosomes in understanding RCC.
Research 1
A seminal study demonstrated the successful isolation and characterization of tissue-derived exosomes from surgically resected clear cell renal cell carcinoma (ccRCC) tissues and adjacent normal renal tissues. This pioneering work revealed that tissue-derived exosomes from tumor tissues are a rich source of disease-specific information.
-
Through comprehensive proteomic analysis, researchers identified over 3,800 proteins within these vesicles.
-
Azurocidin (AZU1) was found to be highly enriched in tissue-derived exosomes.
-
AZU1 was also significantly more abundant in serum exosomes from ccRCC patients, establishing a direct link between the localized tumor environment and systemic markers.
This research didn't stop at discovery; it delved into function. In subsequent in vitro and in vivo studies, the team found that AZU1 carried by tissue-derived exosomes could enter vascular endothelial cells, disrupting their membrane permeability. This discovery sheds light on a potential mechanism by which RCC spreads, as the disruption of the endothelial barrier is a crucial step in metastasis. This exemplifies the power of tissue-derived exosome research: it not only identifies novel biomarkers but also reveals the functional mechanisms underlying disease progression in the native tumor microenvironment.
Research 2
Another groundbreaking study detailed an optimized protocol for isolating exosomes from human kidney tumor and normal kidney tissue.
This work not only provided a robust method for tissue-derived exosome research but also emphasized the necessity of using tissue-specific approaches to overcome the lack of tumor specificity inherent in blood or urine-based exosome studies.
Ready to Advance Your Project? Get a detailed proposal from our specialists.
The Future Direction of RCC Exosome Applications
The study of RCC tissue exosomes points toward a future of transformative applications. By leveraging the unique molecular signatures within these vesicles, researchers can drive significant advancements in diagnostics and therapeutics.
Precision Liquid Biopsy for RCC
Dissecting Disease Mechanisms
Precision Liquid Biopsy for RCC
By correlating the profiles of tissue-derived exosomes with those of systemic exosomes, we can identify highly specific and non-invasive markers for the early detection, disease staging, and therapeutic monitoring of renal cancer. This approach moves beyond general systemic indicators to pinpoint disease activity at its source, enabling earlier intervention and personalized treatment strategies. The ability to track disease progression and therapeutic response through a simple blood test, guided by insights from the tumor microenvironment, is a game-changer.
Dissecting Disease Mechanisms
Tissue-derived exosomes offer an unparalleled opportunity to dissect the intricate molecular mechanisms underlying RCC. By analyzing the specific proteins, nucleic acids (including miRNAs), and lipids encapsulated within these vesicles, researchers can identify key signaling pathways and cellular interactions that drive tumor proliferation, metastasis, and immune evasion. This deep understanding of disease biology is essential for developing novel therapeutic targets. For instance, if a specific exosomal protein is found to promote angiogenesis, it could become a prime target for a new drug.
Turn Your Ideas into Reality. Partner with us to explore the full potential.
Creative Biolabs' Expertise and Services
Creative Biolabs has developed specialized platforms and optimized protocols to address the unique complexities of working with tissue-derived exosomes. Our mission is to empower our clients to unlock the full potential of tissue-derived exosomes.
We offer a comprehensive, end-to-end suite of services tailored specifically for renal cell carcinoma tissue exosome research:
Please contact us now. Our professional scientific team is eager to discuss your specific research ideas and help you explore the mysteries of RCC tissue exosomes.
FAQs
Q: Why are exosomes from RCC tissue a better research tool than those from blood or urine?
A: Exosomes isolated directly from RCC tissue offer a level of specificity and authenticity that systemic biofluid exosomes cannot match. They are secreted by the very cells that constitute the tumor and its microenvironment, meaning their molecular cargo directly reflects the localized pathological changes. In contrast, blood and urine exosomes are a mixed population from various tissues, diluting and potentially obscuring the specific signals from the kidney tumor.
Q: How do you handle the challenges of isolating exosomes from tissue samples?
A: Isolating exosomes from tissue is inherently difficult due to the complex tissue structure and the need to preserve exosome integrity. At Creative Biolabs, we have developed a specialized, optimized protocol that begins with the precise collection and handling of tissue. We use a combination of enzymatic digestion and multi-step purification techniques to efficiently separate exosomes from the tissue matrix while minimizing cellular contamination. Each batch is then meticulously characterized to confirm exosome purity and quality, ensuring reliable downstream results.
Q: How do you ensure the exosomes isolated from tissue are pure and free of contamination?
A: Isolating exosomes from tissue is challenging due to the complex structure and dense extracellular matrix. Our proprietary and optimized methods, including enzymatic digestion, are designed to carefully collect released exosomes while avoiding contamination from free proteins or cellular debris. We then rigorously characterize the isolated vesicles using multiple techniques (e.g., NTA, Western Blotting for specific markers and absence of cellular markers, TEM) to ensure high purity and integrity.
Q: What downstream applications can I perform with the exosomes you isolate?
A: In addition to our comprehensive profiling services, you can use these exosomes for functional studies, such as investigating their role in intercellular communication, immune modulation, or metastasis. We also offer in vitro and in vivo functional verification services to help you validate the biological effects of the exosomes, making our service a one-stop solution for both discovery and mechanistic research.
Q: Can you help me find specific exosome biomarkers for my research project?
A: Yes, absolutely. Our comprehensive exosome profiling services are designed to identify novel biomarkers. By combining our advanced isolation methods with state-of-the-art proteomic detection, RNA sequencing, and lipidomic/metabolomic analysis, we can provide an in-depth molecular signature of the exosomes. Our team of experts will then help you analyze this data to pinpoint specific proteins, nucleic acids, or lipids that correlate with your disease model or research questions, paving the way for targeted biomarker discovery.
Your Vision, Our Expertise. Let's discuss how our services can provide the solutions.
Reference
-
Qin, Zhiyuan, et al. "Extracellular vesicles in renal cell carcinoma: multifaceted roles and potential applications identified by experimental and computational methods." Frontiers in oncology 10 (2020): 724. https://doi.org/10.3389/fonc.2020.00724. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Schematic diagram of the biological features of EVs.1
Fig.1 Schematic diagram of the biological features of EVs.1










