Replication-Competent Adenovirus Assay
Replication-competent adenovirus detection is necessary for adenovirus vector safety evaluation. Armed with an advanced gene therapy platform, Creative Biolabs offers rapid and professional assays for the replication-competent adenovirus detection to ensure the smooth progress of follow-up research.
Adenovirus Vectors
Adenoviruses are a class of double-stranded DNA viruses with an icosahedral nucleocapsid and none lipid envelope. A diversity of adenoviruses has been identified, and over 50 different types of adenoviruses have been found to cause varying degrees of infections in humans. Currently, adenovirus is a potential and excellent viral vector for gene therapy since that (i) high-efficiency in infection of both replicating and non-replicating cells; (ii) ability to transduce a broad range of hosts; (iii) encoding protein expression without integration into the genome of the host cell. One of the most thoroughly studied and extensively utilized adenoviral vectors is adenovirus serotype 5.
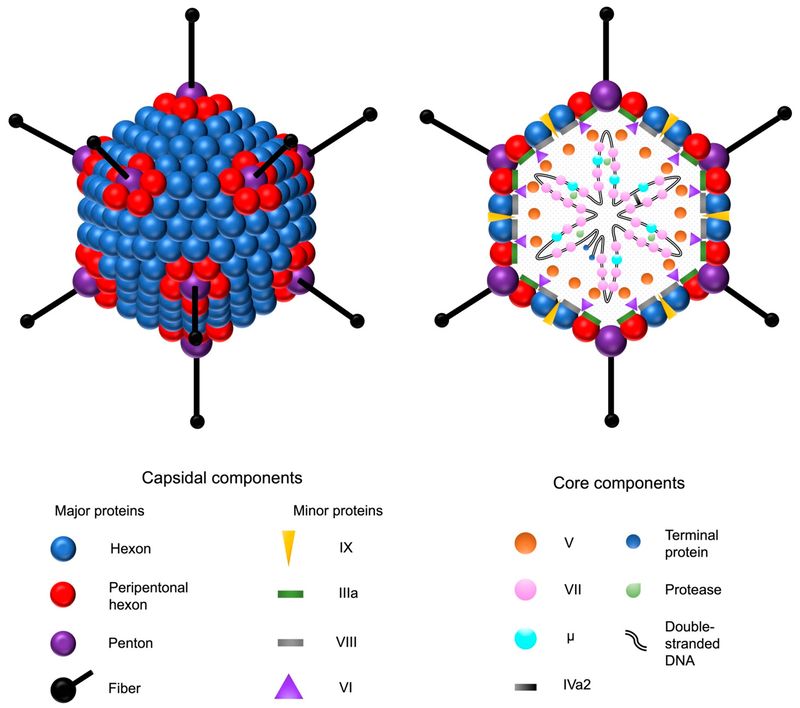 Figure 1 Adenovirus and its structural components.1
Figure 1 Adenovirus and its structural components.1
Types of Adenovirus Vectors
Usually, there are two categories: replication-defective adenoviruses vectors and replication-competent adenoviruses vectors. A replication-competent adenovirus is produced by introducing an exogenous gene to replace the deleted E3 region (a non-essential for viral replication conservative sequence), or replication-defective adenoviruses recombining with host sequences. Replication-competent adenovirus has made great achievements in the fight against cancer as an oncolytic vector.
What is Replication competent Adenovirus?
Replication-competent adenoviruses (RCAs) are a class of adenoviruses that possess a full complement of functional genes required for autonomous replication within host cells. Unlike their replication-defective counterparts, which are genetically engineered to be unable to replicate in normal cells, RCAs can proliferate uncontrollably following infection. This competence stems from the reacquisition of essential viral genes, most notably the E1 region, which is critical for initiating the viral life cycle.
Traditional vs. Modern Replication-Competent Adenovirus Assay Methodologies
The evolution of RCA assay methodologies reflects the industry's demand for higher sensitivity, greater specificity, and faster turnaround times. Traditional methods, while foundational, have been largely supplanted by modern molecular techniques that offer superior performance.
| Methodology | Principle | Pros | Cons |
|---|---|---|---|
| Traditional Plaque Assay | The sample is serially diluted and incubated with a susceptible cell line. The cells are then covered with agar medium. RCA replication causes the surrounding cells to lyse, forming a visible "plaque" | Long-established, direct visualization of infectious units. | Time consuming (typically 1-2 weeks), low throughput, subject to user interpretation, and requires a large sample size. |
| Traditional Co-culture Assay | Mix the sample with the host cell line and culture for a period of time. The presence of RCA is determined by monitoring cytopathic effects (CPE), which are cellular changes indicative of viral replication. | Relatively simple to perform, detects viable virus. | The subjective visual assessment of CPE is very slow (up to 30 days) and may not detect low titer contamination. |
| Quantitative Polymerase Chain Reaction (qPCR) | A highly sensitive method for detecting and quantifying specific RCA DNA sequences. It uses fluorescent probes to monitor DNA amplification in real time. | High sensitivity and specificity, fast turnover speed (usually 1-3 days), quantifiable results, and high throughput. | Detects DNA but cannot distinguish between replication-competent and replication-defective particles unless prior to a pre-amplification step. |
| Digital Polymerase Chain Reaction (dPCR) | The new generation of technology divides the sample into thousands of individual reactions. Each partition calculates the number of amplifications of the target DNA, providing absolute quantification. | Unparalleled sensitivity, no need for absolute quantification of standard curves, very suitable for low-level detection, and has strong resistance to inhibitors. | Higher cost of specialized equipment. |
Replication-Competent Adenovirus Assay
Replication-competent adenovirus has been extensively studied and applied in antineoplastic gene therapy, but there are also unsafe factors due to their ability to replicate. More importantly, replication-competent adenovirus increases with each successive amplification, and the risk of infection increases accordingly. Thus, sensitive assays for the detection of replication-competent recombinant adenoviruses are necessary to adenovirus vector characterization. We provide professional replication-competent adenovirus detecting assays, including but not limited to:
- Cytopathic Effect Detection Assay: observation and analysis of the cytopathic effect following the infection and amplification of recombinant adenovirus vectors.
- PCR: This method combines microcarrier cell culture with polymerase chain reaction (PCR) to rapidly detect replicative adenovirus. Adenoviral vectors are incubated on A549 cells in a microcarrier cell culture system, and the amplified replicative adenovirus is detected using E1-specific quantitative PCR.
- Other Detection Assays: combination replication-competent adenovirus amplification with immunocytochemistry staining, cytopathic effect evaluation followed by differential amplification of adenoviral vector, etc.
Applications of Replication-Competent Adenovirus Assay
-
Gene Therapy
For E1-deleted adenoviral vectors carrying therapeutic genes, RCA testing is mandatory for batch release. It provides a critical safety checkpoint, ensuring that the vector received by patients can deliver the gene as intended and does not cause unwanted viral infection. This is particularly important for treating rare genetic diseases, as the patient population is often pediatric or immunocompromised. -
Oncolytic Virotherapy
Oncolytic viruses are designed to selectively replicate in and kill cancer cells. Here, RCA testing plays a different but equally important role. It is used to demonstrate the genetic stability of the oncolytic virus and confirm that it does not revert to a wild-type, systemically pathogenic strain. This test verifies tumor-specific viral replication, a cornerstone of its therapeutic mechanism and safety. -
Vaccine Development
Adenoviral vectors are also widely used as vaccine platforms. In this context, RCA testing ensures that vaccine products, designed to present antigens to the immune system, are free of any replication-competent virus, thereby preventing the vaccine itself from causing disease.
Core Services at Creative Biolabs
As a leading company in viral vector construction, Creative Biolabs provides a series of replication-competent adenovirus detection services. Moreover, our scientists, specialized in gene therapy, has successfully established a comprehensive and perfect gene therapy services system aiming to offer rapid and one-stop viral vector-based gene therapy services to customers all over the world.
Our Workflow
Our streamlined workflow is designed for maximum efficiency and transparency.
- Initial Inquiry and Consultation: A dedicated project manager will discuss your specific needs, sample types, and regulatory requirements.
- Protocol Design: We develop a customized assay based on your vector construct and regulatory pathway.
- Sample Submission: You ship your samples to our certified laboratory facilities.
- Assay Execution: Our experienced scientists perform RCA assays using our validated, highly sensitive methods.
- Data Analysis and Reporting: We meticulously analyze the raw data and generate a comprehensive report that includes all raw data, a detailed methods section, and a clear pass/fail conclusion.
Customer Review

"The team at Creative Biolabs provided us with an exceptionally fast and accurate RCA assay. Their dPCR-based approach detected low-level contamination that our previous supplier had missed. Their regulatory expertise and timely communication were invaluable. We now rely on them for all our viral vector quality control needs."
- Dr. Alistair Finch, Head of Manufacturing
Frequently Asked Questions
Q: How do you validate that your assay detects all potential RCA variants?
A: Our assay validation includes testing against a panel of well-characterized RCA variants generated through different recombination mechanisms. We use bioinformatic analysis of vector and production cell line sequences to identify potential recombination hotspots and ensure that our assay targets conserved regions present in all predicted RCA variants. For customer-specific assays, we include inclusive testing using RCA spikes relevant to their specific system.
Q: Can you test RCA in adenoviral vectors with non-E1 deletions (such as E3 deletions)?
A: Yes, we offer customized RCA testing solutions for non-E1 deletion adenoviral vectors.
Adenoviral vectors with non-E1 deletions (such as E3 deletions, E4 deletions, or multigene deletions) require tailored RCA testing strategies because their replication defects and recombination risks differ from those of classic E1 deletion vectors. Our team has extensive experience adapting RCA testing for these systems, supported by validated protocols and real-case data.
Q: How much sample is required for RCA testing?
A: For most molecular assays, we require approximately 10^9 viral particles for comprehensive testing. Smaller quantities may be sufficient for screening assays, and we can discuss this based on your specific constraints. For cell-based assays, larger sample sizes may be required due to the serial channeling approach.
Q: How long does it typically take to complete an RCA assay?
A: Our standard qPCR/dPCR assays provide results within 5-7 business days of sample receipt. Due to the required incubation period, cell-based CPE assays require 14-21 days. A rapid turnaround option is available for time-sensitive projects, providing qPCR results in as little as 3 business days.
Q: Can you support RCA testing for novel adenoviral serotypes other than Ad5?
A: Of course. We have experience with a wide range of adenoviral serotypes and custom vectors. Our approach involves initial bioinformatics analysis to identify appropriate assay targets, followed by careful vector construct-specific analysis, design, and validation. We welcome the opportunity to work with novel adenoviral platforms.
Connect with Us Anytime!
At Creative Biolabs, we understand that high-quality RCA testing is integral to our clients' success. Our services are built on deep scientific expertise, state-of-the-art technology, and a commitment to regulatory excellence. Our team of PhD-level scientists, with extensive experience in virology and quality control, ensures our protocols are meticulously designed and validated according to the latest EMA, and ICH guidelines. Please contact us to discuss your demands or to request a proposal.
Reference
- Leikas A J, Ylä-Herttuala S, Hartikainen J E K. Adenoviral gene therapy vectors in clinical Use—Basic aspects with a special reference to Replication-Competent adenovirus formation and its impact on clinical safety. International Journal of Molecular Sciences, 2023, 24(22): 16519. https://doi.org/10.3390/ijms242216519 (Distributed under Open Access license CC BY 4.0, without modification.)
