Capsid-modified Adenovirus Vector Construction
The adenovirus had been sequenced entirely and the detailed mechanism of how this virus replicates and assemble is well known. Developing cell-targeting adenoviral vectors has been an effective means of transferring genes in vivo. As a professional supplier in the biological field, Creative Biolabs has new approaches to produce a modified viral genome by genetically manipulating the capsid genes of adenoviral vectors. We provide end-to-end solutions for capsid-modified vector construction services and our valuable toolboxes can overcome some limits of time-consuming and multiple steps for the easy, rapid construction of capsid-modified adenoviral vectors.
Adenovirus Vector Introduction
Adenovirus (AdV) vectors hold a cornerstone position in gene therapy and vaccinology due to their high transduction efficiency, ability to infect both dividing and non-dividing cells, and good safety profile. As versatile biological delivery vectors, AdVs can carry large gene payloads (up to 7.5 kb for E1/E3 deletion vectors) and achieve transient, high-level transgene expression without integrating into the host genome. However, their clinical application potential is typically limited by two major biological barriers: firstly, the inherent tropism determined by the ubiquitous Coxsackievirus receptor (CAR); and secondly, high pre-existing immunity against common serotypes (especially Ad5).
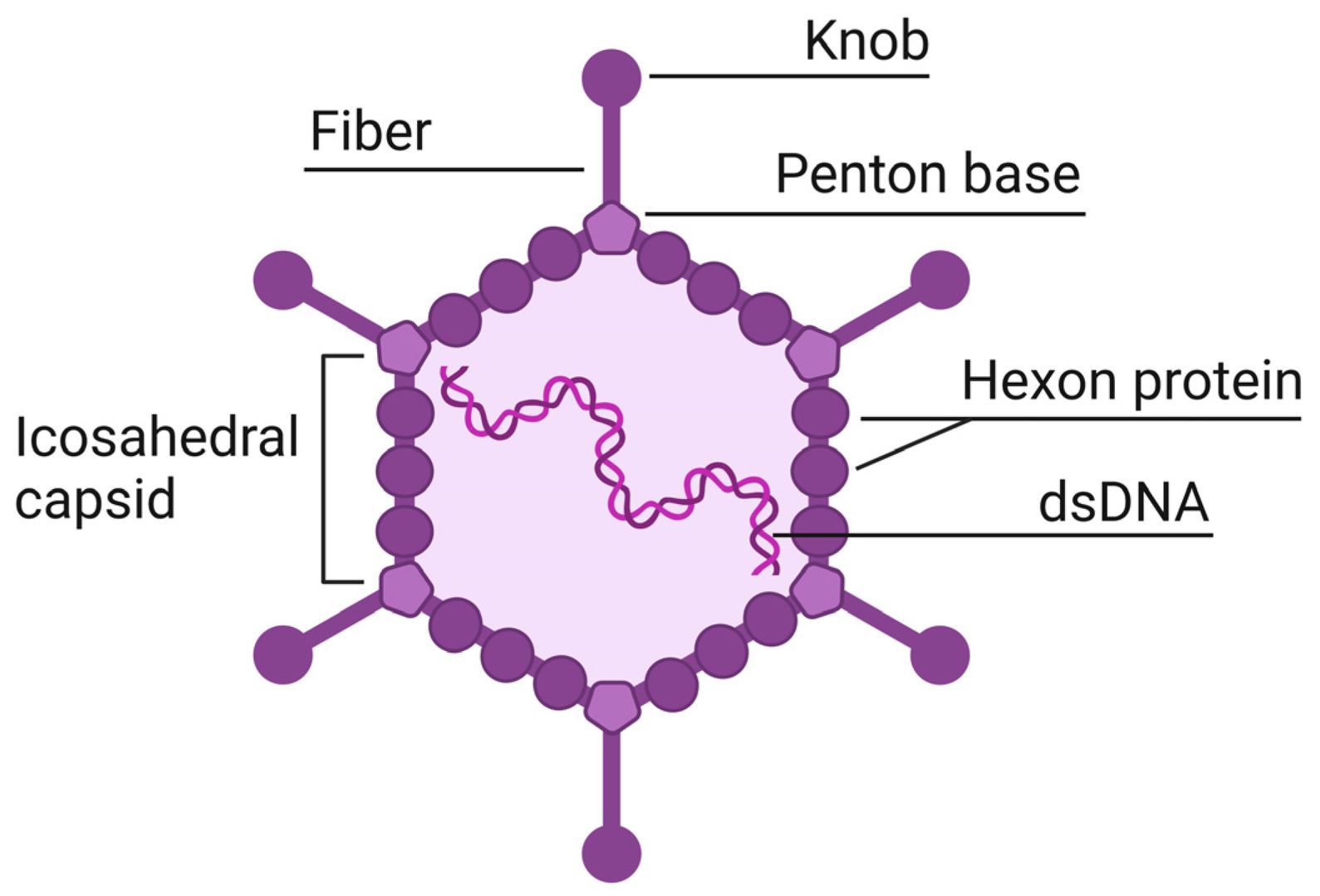 Figure 1. Structure of adenovirus.1
Figure 1. Structure of adenovirus.1
Capsid-Modified Adenovirus Vector Construction
Capsid-modified adenovirus vectors represent the forefront of viral vector engineering, focusing on the strategic modification of viral protein coats to overcome their natural limitations and enhance therapeutic potential. The adenovirus capsid is composed of three main proteins—hexon, penton base, and fiber—each offering unique opportunities for vector modification. Through rational design and directed evolutionary approaches, researchers can construct custom vectors with enhanced functions to meet specific research application needs.
Key goals of capsid modification include:
- Retargeting the vector to specific tissues or cell types
- Reducing immunogenicity for repeated dosing
- Bypassing pre-existing immunity in the population
- Improving transduction efficiency for refractory cell types
Key Capsid Proteins and Their Modification Potential in Adenovirus Vectors
| Capsid Protein | Structural Role | Modification Approaches | Primary Impact |
|---|---|---|---|
| Hexon | Forms hexagonal capsid surface | Hypervariable region swapping, peptide insertions | Altered immunogenicity, liver detargeting |
| Fiber | Projects from vertices | Domain swapping, genetic fusions | Altered receptor binding, retargeting |
| Penton | Base of fiber projections | RGD modifications, ligand incorporation | Enhanced internalization, altered tropism |
Capsid-Modified Adenovirus Vector Application
Adenovirus Vector Vaccines
Capsid-modified adenoviruses (AdVs) have become a globally recognized, highly effective vaccine platform, particularly suitable for emerging infectious diseases. Viral vectors can deliver antigen-coding sequences directly to host cells, triggering strong T-cell and B-cell-mediated immune responses.
Oncolytic Virus Therapy
Engineered adenoviruses are a promising class of oncolytic viruses capable of selectively replicating and destroying cancer cells. Transcriptomic studies using patient-derived organoids have identified gene expression profiles associated with sensitivity to oncolytic viruses, including adenovirus vectors.
Gene Therapy in Genetic Diseases
Capsid-modified adenoviruses can target therapeutic genes to affected tissues while minimizing off-target effects. Recent advances in capsid engineering have improved transduction efficiency in tissues previously difficult to transfer genes, expanding potential treatment options for monogenic diseases.
Cell Engineering and Immunotherapy
Adenoviruses modified using "molecular glue" technology have demonstrated remarkable efficacy in modifying primary B-cells and T-cells for adoptive immunotherapy. This method can easily and effectively target adenovirus vectors to specific immune cell populations, thereby enhancing gene transfer in vitro and in vivo.
Our Service
Gene transfer via adenovirus-based vectors has widely been used in human gene therapy and clinical trials. The reduction of adenovirus vector-associated innate immune toxicities will greatly broaden the utility of this bio-platform for use in multiple medical applications. At Creative Biolabs, we have developed novel techniques and integrated services to achieve the modification of adenoviral capsids leading to high-level gene expression. And our scientists with years of experience ensure the safety and efficacy of these vectors in targeting a variety of diseases.
Knobless Adenovirus Vector Construction Service - The knobless fibers are prototype substrates for versatile addition of targeting ligands to produce truly targeted adenovirus. Our approach to develop targeted adenovirus is to delete the intact fiber knob and replace it with two different protein moieties.
Chimeric Adenovirus Vector Construction Service - The circulating antibodies to adenovirus capsid proteins is a barrier for adenovirus delivery system used in gene therapy. We made efforts to engineer the hexon to evade anti-hexon antibodies by generating chimeric adenoviruses harboring hexons from other serotypes. The constructed vectors rarely cause human infections or use adenoviruses from non-human sources, such as canine, bovine, or ovine.
Peptide-incorporated Adenovirus Vector Construction Service - We provide a popular method of adenovirus targeting by directing the vector towards different cell receptors. Redirecting can be achieved by linking custom-made peptides specifically to cell surface proteins via genetic integration, chemical peptide conjugation or bridging with bifunctional adapter molecules.
Antibody-modified Adenovirus Vector Construction Service - We have developed strategies to alter adenovirus tropism to make feasible cell-specific targeting by using molecular adapter proteins and genetic capsid modifications. The molecular adapters consist of chemically coupled antibody-ligand fusions, diabodies, as well as genetic fusions between ligand or scFvs and the ectodomain of coxsackie adenovirus receptor.
Adenovirus Vector Production and Purification
Highly efficient, high-titer production is crucial for large-scale preclinical studies. The industry gold standard is vector amplification using the HEK293 cell line (or its variants, such as HEK293T or PER.C6) via triple transfection.
Production
- Transfection: Transfer plasmid, helper plasmid, and backbone plasmid are co-transfected into HEK293 cells.
- Amplification and Lysis: Cells are cultured until cytopathic effect (CPE) is observed, leading to cell lysis and release of viral particles.
- Collection: Cell lysates containing crude viral particles are collected.
Purification and Quality Control (QC)
The purity, integrity, and concentration of the vector are critical for safety and efficacy.
- Preliminary Clarification: Cell debris is removed by centrifugation and filtration. Chromatography (High-Yield Processing): We employ state-of-the-art chromatographic methods (e.g., anion exchange chromatography or affinity chromatography using a monolithic column) for preliminary purification, separating empty capsids from intact capsids. This method offers superior scalability and reproducibility compared to conventional methods.
- Ultrapurification (Traditional High Purity): For materials, we typically employ isodense cesium chloride (CsCl) density gradient ultracentrifugation to ensure the highest degree of separation between infectious (intact) particles and non-infectious (empty) particles. Separation is measured by the particle infection unit (P/IU) ratio.
- Sterile Filtration and Formulation: The final carrier is buffered (typically using glycerol or sucrose solution to improve stability) and sterile filtered.
Why Choose Our Services?
With unique technical expertise, advanced platforms, and a commitment to customer success, Creative Biolabs is the preferred partner for capsid-modified adenovirus vector development.
-
Integrated Platform
Our comprehensive solutions cover every stage from initial design to final validation, providing a one-stop shop for all your adenovirus vector needs. -
Technical Expertise
Our team comprises virologists, structural biologists, and synthetic biologists with deep knowledge of adenovirus biological strategies.
-
Quality Commitment
We implement rigorous quality control at every stage of the process to ensure our clients receive vectors with the highest purity, potency, and stability. -
Win-Win Partnership
We go beyond traditional supplier-customer relationships, building true scientific partnerships with dedicated project teams.
-
Scalable Production Capacity
We have standard dedicated facilities equipped with scalable bioreactor systems, enabling us to produce vectors of various grades. -
Proven Success
Our capsid-modified vectors have demonstrated efficacy in challenging in vivo models, including cross-protection against multiple influenza strains in vaccine applications.
Frequently Asked Questions
Q: What are the main advantages of capsid-modified adenovirus vectors compared to unmodified vectors?
A: Capsid-modified adenovirus vectors offer several significant advantages, including enhanced target cell specificity, reduced off-target transduction, evasion of pre-existing immunity, and improved safety. These modifications enable more precise gene delivery, potentially higher therapeutic efficacy, and the possibility of repeated dosing—limitations of traditional adenovirus vectors.
Q: Can your capsid modifications accommodate large targeting ligands?
A: Yes, our platform supports the integration of various targeting motifs, including single-chain antibodies and other protein ligands. Molecular glue technology is particularly suitable for large ligands because it minimizes structural constraints on the capsid while ensuring precise orientation of the targeting domain.
Q: How do you validate the targeting specificity of your modified vectors?
A: We employ a comprehensive validation methodology, including in vitro transduction experiments using relevant cell lines, primary cells, and mixed cell populations; flow cytometry analysis of cell type-specific transduction; and in vivo biodistribution studies in appropriate animal models. For vectors with immunotherapeutic potential, we also conduct functional assays to confirm their effective activation of immune cells.
Connect with Us Anytime!
Gene therapy is an innovating and appealing strategy for the treatment of many human diseases, which makes use of vehicles for delivering therapeutic genes into the body of patients. The adenovirus with a non-enveloped protein capsid has gained much attention as an efficient vector for gene transfer. As a reliable partner in the gene therapy market, Creative Biolabs provides a diversity of capsid-modified adenovirus vector construction services to satisfy clients' special requirements. For more information, please feel free to contact us.
Reference
- Muravyeva A, Smirnikhina S. Strategies for modifying adenoviral vectors for gene therapy. International Journal of Molecular Sciences, 2024, 25(22): 12461. https://doi.org/10.3390/ijms252212461 (Distributed under Open Access license CC BY 4.0, without modification.)
