AAV Titration Assay
Creative Biolabs is able to provide state-of-art services for any adeno-associated virus (AAV) vector project with qualitative measurements. Our professional team will provide flexible solutions for AAV vector generation, titration testing, and safety evaluation. We are also specialized in improving current AAV vectors for gene therapy, which can boost long term efficacy and reduce the cost to a more affordable level.
Adeno-associated Virus (AAV) Titration Introduction
Adeno-associated virus (AAV) titration is a critical process for accurately quantifying viral vectors used in gene therapy, ensuring precise administration, production consistency, and patient safety. It involves evaluating physical titers (total viral particles, regardless of function) and infectious titers (functional, transduced particles). Physical titers are typically determined by quantitative vector genome copy qPCR and ddPCR, as well as ELISA for measuring total capsid proteins. For infection titers, cell-based infectivity assays such as reporter gene assays or TCID50 are used to measure functional units. Understanding the frequent differences between these titers is crucial as it reflects the proportion of empty or non-functional capsids. Strict titration is essential for preclinical research, clinical development, and regulatory compliance.
Methods of Adeno-associated Virus Titration Determination
A wide collection of strategies has been developed to detect the titration of produced vectors, such as quantitative PCR (qPCR), enzyme-linked immunosorbent assay (ELISA), as well as dot-blot assays. Pilot studies have shown that ELISA can be widely used to detect the number of viral capsids that contain genetic materials. The data suggest that it is an accurate and reproducible way for AAV vector titration. Meanwhile, scientists have revealed that dot-blot and qPCR are reliable methods for determining the titration of different AAV vectors, among them, qPCR is the most rapid and high-throughput assay to titer AAV vectors in pre-clinical studies. For instance, more recent studies have reported that the infectious titers of recombinant AAV-2 (rAAV-2) particles can be identified by a novel qPCR assay. Moreover, this method has been validated by using the infectious center assay (ICA) and flow cytometry (FACS) assay.
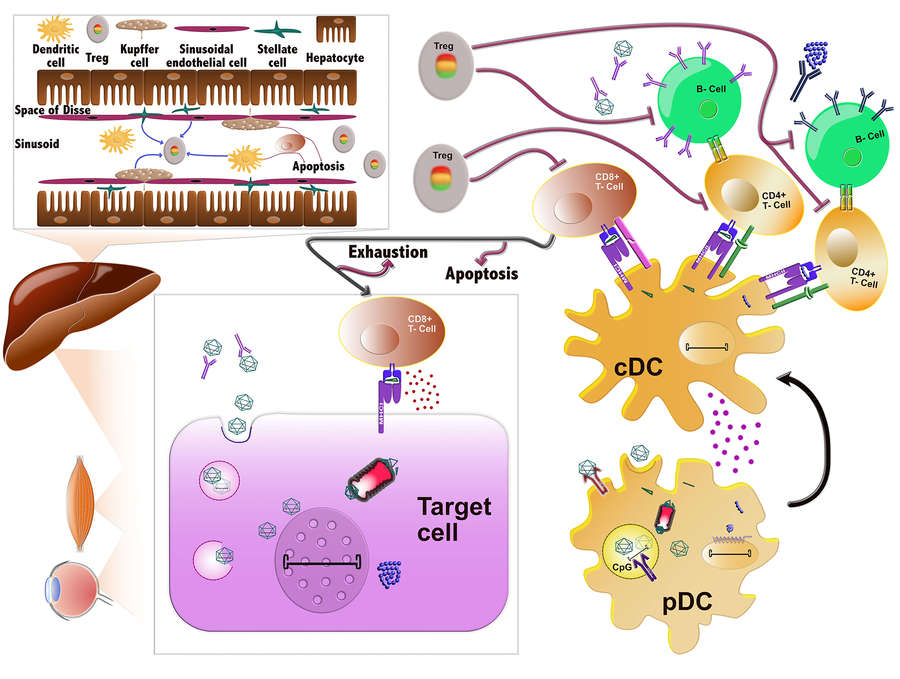 Figure 1 Immune responses against AAVs.1
Figure 1 Immune responses against AAVs.1
If there is no precise titration, you will face the following risks:
- Incorrect dosage: Insufficient dosage may result in ineffective treatment, while excessive dosage may lead to potential toxicity and unnecessary immune reactions.
- Lack of repeatability: Inconsistent titration makes it difficult to replicate experimental results, which is a major obstacle to research and regulatory approval.
Core Services at Creative Biolabs
Creative Biolabs is able to provide state-of-art services for any AAV vector project with qualitative measurements. Our professional team will provide flexible solutions for AAV vector generation, titration testing, and safety evaluation. We are also specialized in improving current AAV vectors for gene therapy, which can boost long term efficacy and reduce the cost to a more affordable level.
-
Performing AAV vector genome titration through qPCR
using industry standard qPCR protocol for high-throughput, sensitive, and reliable quantification of AAV vector gene copies. We use optimized primer probe sets for various AAV serotypes and transgenic sequences. -
Performing AAV vector genome titration through ddPCR
Absolute quantification of AAV vector genes with extremely high accuracy and reproducibility, particularly suitable for high-precision critical samples or low copy number detection. -
AAV capsid ELISA for total particle quantification
Rapid and specific quantification of total AAV capsid proteins, providing basic measurements for assembling virus particles (loaded and unloaded). -
AAV infection unit titration
Functional evaluation of AAV vectors using highly sensitive cell lines and optimized reporter gene systems (such as GFP, luciferase). We provide robust IU assays, which are crucial for evaluating carrier efficacy.
Capsid Titer Determination: Quantification of Total Viral Particles (VP)
To quantify the total number of assembled AAV capsids, we utilized enzyme-linked immunosorbent assay (ELISA). AAV capsid ELISA is a sandwich immunoassay method. Coat the conformational epitope specific capture antibodies on the assembled AAV capsid onto a microtiter plate. AAV particles in the sample bind to capture antibodies. The secondary detection of antibodies is usually biotinylated and then binds to different epitopes on the captured AAV, followed by streptavidin HRP conjugates. Subsequently, a colorimetric substrate reaction occurs, and the absorbance is proportional to the number of intact capsids.
Workflow of Our Adeno-associated Virus Titration
01 Plate Coating: The microtiter plate is pre coated with serotype specific capture antibodies.
02 Sample Incubation: Add diluted AAV samples and serotype matched AAV standards into wells.
03 Detection: After washing, add biotinylated detection antibodies, and then add streptavidin HRP.
04 Color Development: Adding substrate will cause color development, which can be measured by spectrophotometry.
05 Quantification: The standard curve generated based on known AAV capsid concentration can accurately determine total viral particles (VP/mL).
Why choose Creative Biolabs to meet your AAV titration
Professional Knowledge
A team of doctoral level scientists with extensive experience in virology, gene therapy, and molecular biology.
Cutting Edge Technology
access to advanced instruments and validated protocols for all major titration methods.
Quality Assurance
Strictly adhere to quality control measures and validated SOPs to ensure reliable and reproducible results.
Fast Turnaround Time
An efficient workflow designed to provide timely and accurate results, supporting your project schedule.
Customer Support
Provide specialized project management and scientific support throughout the entire collaboration process.
Frequently Asked Questions
Q1: What is the fundamental difference between physical titer and infectious titer?
A1: Physical titers quantify the total number of viral particles (e.g. vector genome copies or assembled capsids), regardless of their ability to infect cells. On the contrary, the infection titer only measures functional active particles that can successfully transduce target cells and express transgenes.
Q2: Why is it important to measure both physical and infectious titers?
A2: These two indicators are crucial for comprehensive AAV characterization. The physical titer is important for manufacturing consistency and yield evaluation, while the infection titer is more directly related to the biological activity and therapeutic potential of the carrier in the patient's body. Comparing the two can help determine the ratio of fully loaded and unloaded jackets and the overall carrier mass.
Q3: Why might my physical titer and infectious titer results not match?
A3: Differences are typically caused by the presence of empty capsids (which do not contain the genome but contribute to physical titers), partially packaged genomes, or damaged/aggregated viral particles. These factors will increase the body's titer, but will not affect the infection titer. Differences in detection conditions, cell line sensitivity, and operator variability may also play a role.
Q4: Which AAV serotypes can Creative Biolabs titrate?
A4: We have extensive experience in various AAV serotypes, including but not limited to AAV1, AAV2, AAV3, AAV4, AAV5, AAV6, AAV7, AAV8, AAV9, AAVrh10, and novel synthetic capsids. We can also develop customized titration analysis for less common or proprietary serotypes.
Q5: What types of samples do you accept for AAV titration?
A5: We accept various AAV containing samples, including crude lysates, purified virus precursors, conditioned media, and samples for in vivo studies (such as tissue homogenates, CSF). Please consult our technical support team for specific sample preparation and shipping instructions.
Connect with Us Anytime!
Creative Biolabs' integrated workflow provides a powerful solution for the comprehensive characterization of AAV vectors, which is crucial for ensuring the quality, safety, and effectiveness of gene therapy products. Please feel free to contact us for more details and our scientists will conduct further in-depth discussion on your project.
Reference
- Rapti K, Grimm D. Adeno-associated viruses (AAV) and host immunity–a race between the hare and the hedgehog. Frontiers in immunology, 2021, 12: 753467. https://doi.org/10.3389/fimmu.2021.753467 (Distributed under Open Access license CC BY 4.0, without modification.)
