Plaque-Forming Titer Assays
Creative Biolabs has consistently been at the forefront of biotechnology advancement, offering exceptional service quality. To better serve our customers, we have launched plaque-forming titer assays designed to monitor and ensure the quality of viral vectors. These plaque assays can be utilized to purify viral clonal populations and measure viral titers expressed as plaque-forming units per milliliter (pfu/ml). This precision allows you to accurately regulate the viral load used for infecting cells in your subsequent experiments.
Viral Titer Introduction
Analyze multiple dilutions of reserve samples to determine one or more dilutions that produce a countable number of plaques. At the lowest dilution, too many infectious particles can destroy large cell monolayers or produce too many overlapping and indistinguishable plaques. At the highest dilution, there may be no plaques at all. At the optimal dilution, plaques are counted to determine the titer of the original reserve sample, typically reported as units of plaque formation per milliliter (PFU/ml).
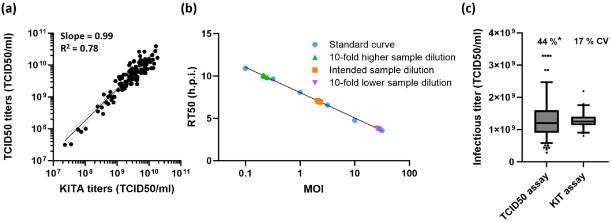 Figure 1 Performance characteristics of the kinetic infectious virus titer assay.1
Figure 1 Performance characteristics of the kinetic infectious virus titer assay.1
PFU/ml vs IU/ml
The design of this detection ensures that each plaque is infected by reproducing a single infectious viral particle. Therefore, PFU/ml is considered a measure of the number of infection units per milliliter (IU/ml), but it should be noted that the ratio of plaques to infection particles in the applied aliquots cannot be determined as one-to-one. In addition, please note that the titer of the sample depends on the measurement conditions used to determine it, as infectivity is influenced by many factors such as the type of host cell, pH value, and culture medium. By changing key measurement parameters, titers can differ by several orders of magnitude.
Plaque Formation
Plaque detection requires the cultivation of cells that are susceptible to infection by the virus of interest. Firstly, the cells are seeded onto a surface where they can adhere and grow, and then left overnight to form a fused monolayer (a sticky cell sheet covering the entire growth surface). Then dilute the virus sample several times and add aliquots of each dilution solution into culture dishes or cell wells. The incubation period allows the virus to attach to target cells before removing the inoculum. Then cover the culture with a medium containing nutrients and substances (such as agarose or methyl cellulose) to form a gel or semi-solid coating. Infectious viral particles that enter cells and replicate can trigger the release of progeny viral particles. The gel restricts the movement of particles, so the newly generated virus can only infect adjacent cells. If the virus kills infected cells, the dead (or dying) cells will separate and form a hole in the monolayer through lysis or other means. This space without cells now is called a plaque, which appears as circular spots on the growth surface.
 Figure 2 Schematic description of the test steps for determining the rVSV-SARS-CoV-2-S concentration in Vero E6 cell culture using the plaque assay.2
Figure 2 Schematic description of the test steps for determining the rVSV-SARS-CoV-2-S concentration in Vero E6 cell culture using the plaque assay.2
Core Services at Creative Biolabs
Custom Plaque Assay Development and Optimization
We specialize in developing and optimizing plaque detection conditions for various cell lines and viral systems, ensuring maximum sensitivity and reproducibility even for challenging or novel viruses. Our expertise minimizes the time and labor typically associated with testing settings.
Stringent Quality Control and Reproducibility
Our plaque testing is conducted under strict quality management measures to ensure unparalleled accuracy, precision, and compliance with industry standards. We strictly address the potential subjectivity in counting through standardized protocols and advanced imaging techniques.
Versatile Viral Vector Plaque Titering
We specialize in plaque formation titer determination for various viral vector platforms, including adeno-associated virus (AAV), adenovirus (AdV), lentivirus, retrovirus, and other customized viral vector systems, to ensure accurate dosage for gene therapy applications.
Plaque Reduction Neutralization Test (PRNT) Services
We provide comprehensive PRNT services for accurate assessment of neutralizing antibody titers, which are crucial for vaccine efficacy research and serological diagnosis, directly utilizing the robustness of plaque detection.
Plaque Assay Service Protocol
- The customer determines the number of plates used in the experiment and the dilution requirements for each plate.
- The corresponding host cells required for viral infection are inoculated on the plates and cultured until the confluency of the cells reaches 70%.
- Prepare the agarose solution, we will prepare the corresponding volume of agarose solution according to the customer's needs, and strictly control the process to avoid pollution.
- Prepare virus dilutions corresponding to the plate settings required by the customer.
- Inoculate monolayer cells with 1 ml of the appropriate virus dilution, incubate for 1-2 hours to allow the virus to adsorb.
- Monolayer cells are covered with agarose gel and incubated for 5-10 days to observe cell infection. If dyeing is required, we will add the corresponding dye to the prepared agarose.
- The well-isolated plaques on the plate are counted to determine the titer of the virus.
Frequently Asked Questions
Q: What is the difference between TCID50 assay and plaque assay?
A: They are used to measure infectious virus, but in different ways:
- Plaque Assay: This is the gold standard for directly counting infectious virus as Plaque-Forming Units (PFU/mL). Each PFU comes from a single infectious virus that forms a plaque, a visible clear zone in a layer of cells, where virus spread is limited by a semi-solid agar or carboxymethyl cellulose (CMC) overlay.
- TCID50 Assay: This assay estimates the Tissue Culture Infectious Dose 50 (TCID50/mL), the dose of virus that causes a cytopathic effect (CPE) in 50% of the cell cultures in a well of a plate in a liquid medium. The assay is statistically based, and is a faster and more general method than a plaque assay for viruses that do not form plaques well or at all. It is less direct than a plaque assay.
Q: What are some factors that can influence the accuracy of a plaque assay?
A: Several factors can impact the accuracy of a plaque assay, including: the health and confluency of the host cell monolayer, the accuracy of serial dilutions, the uniformity of virus adsorption, the composition of the overlay medium, the incubation time and conditions, and the staining and counting methods used. Any variability in these steps can affect the results.
Q: Why is an overlay medium used in a plaque assay?
A: The overlay medium is used to restrict the diffusion of progeny virions away from the initially infected cell. This ensures that secondary infections occur only in neighboring cells, resulting in localized cell lysis and the formation of discrete, countable plaques, each arising from a single infectious unit.
Q: What is the main difference between plaque detection for virus quantification and qPCR?
A: Plaque test measures infectious virus particles (PFU), directly indicating biological activity. QPCR quantification of viral nucleic acids (such as genome copies), regardless of whether the particles are infectious or intact. Although qPCR is faster and more sensitive, it cannot replace plaque detection to determine infectivity.
Customer Review of Plaque-Forming Titer Assays
"Collaborating with Creative Biolabs for our viral vector characterization has been a game-changer. Their team's expertise in plaque-forming titer assays is truly exceptional. We needed precise quantification of our novel AAV constructs, and their optimized protocols delivered highly reproducible and accurate results, even for our challenging serotypes. The detailed reports and rapid turnaround time significantly accelerated our gene therapy research. Their customer support was also fantastic, providing clear communication every step of the way. Creative Biolabs is now our go-to partner for all our infectious titer needs."
— Dr. Elara Vance, Lead Scientist
Get in Touch Today!
Designing and constructing adenoviral vectors for the delivery and expression of foreign genes according to the needs of customers has always been a superior service project of Creative Biolabs. In order to help customers more accurately control the amount of virus in the experiment, we provide customers with plaque-forming titer assay, as well as virus plaque purification, plaque virus amplification and other services established on this basis. If you have any questions or have any difficulties, you can contact us by email or send us an inquiry to find a complete solution.
References
- Hotter D, Kunzelmann M, Kiefer F, et al. High-throughput determination of infectious virus titers by kinetic measurement of infection-induced changes in cell morphology. International Journal of Molecular Sciences, 2024, 25(15): 8076. https://doi.org/10.3390/ijms25158076
- Toister E, Cherry L, Lupu E, et al. Development and Validation of a Plaque Assay to Determine the Titer of a Recombinant Live-Attenuated Viral Vaccine for SARS-CoV-2. Vaccines, 2024, 12(4): 374. https://doi.org/10.3390/vaccines12040374
- (Distributed under Open Access license CC BY 4.0, without modification.)
