Lentiviral Vectors Titration Service
Lentiviral vectors (LVs) are efficient vehicles for stable gene transfer in both dividing and non-dividing cells, which makes them a powerful tool in molecular research. However, the use of lentiviruses in research and clinical trials requires precise and validated titration methods. Creative Biolabs has developed a variety of lentiviral titer platforms and proprietary technologies. With in-depth expertise in this field, we provide lentiviral vectors titration service with robust methods.
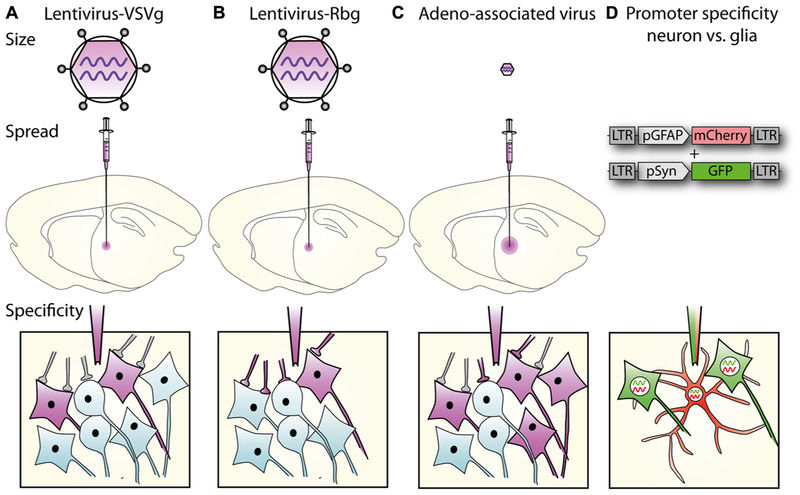 Figure 1 Lentiviral vector spread, transduction, and expression is mediated by particle size, envelope properties and promoter usage.1
Figure 1 Lentiviral vector spread, transduction, and expression is mediated by particle size, envelope properties and promoter usage.1
Lentiviral Vectors
LVs represent a breakthrough in gene delivery technology, derived from complex retroviruses such as the human immunodeficiency virus (HIV-1). These engineered vectors have revolutionized gene therapy and basic research by offering an unparalleled ability to stably deliver genes to both dividing and non-dividing cells. Unlike simpler viral vector systems, LVs possess the unique ability to integrate their genetic payload into the host genome, promoting long-term expression of the transgene and its persistence during cell division.
Table 1 Comparison of Major Viral Vector Systems
| Vector Type | Max Capacity | Integration | Target Cell Type | Key Advantages |
|---|---|---|---|---|
| Lentivirus | ~8 kb | Yes (random) | Dividing & non-dividing | Stable long-term expression, broad tropism |
| γ-Retrovirus | ~8 kb | Yes (random) | Dividing cells only | Simple system, clinical experience |
| Adenovirus | ~8-30 kb | No | Dividing & non-dividing | High titer, well-characterized |
| AAV | ~4.7 kb | No (episomal) | Dividing & non-dividing | Low immunogenicity, excellent safety profile |
Lentiviral Vectors Titration Introduction
LVs are efficient vehicles for stable gene transfer in both dividing and non-dividing cells, such as neurons or hepatocytes, and allow long-term expression of transgenes and target specific cell types via simple pseudotyping processes. Based on these advantages, LVs have often been used to deliver genes of interest into mammalian cells in molecular research and preclinical/clinical trials. However, in order to apply lentiviral methods in research and clinical studies, it firstly requires an assessment of titers. Viral titer determination is an essential quality assessment for lentivirus-based basic research and clinical application, especially the accuracy of this assessment is of the utmost importance for therapeutic dosing of patients. To date, lentiviral titration can be determined by two general categories: nonfunctional (physical titer) and functional (infectious titer) methods.
Nonfunctional Titration Methods
Nonfunctional titration methods include detection of expressed virus proteins (such as p24) or their transcripts using enzyme-linked immunosorbent assay (ELISA), assessment of the reverse transcriptase (RT) activity and determination of the genomic RNA concentration in vector preparations by semi-quantitative northern blotting, dot blot analysis or real-time quantitative polymerase chain reaction (RT-qPCR). LVs are traditionally produced by transient transfection of human embryonic kidney (293T) cells with high concentrations of different plasmids. In general, the nonfunctional titration methods are carried out on the concentrated vector after production.
Functional Titration Methods
For clinical studies and other gene therapy applications, it is essential to accurately determine the functional titers of LVs. Functional titer, or infectious titer, is defined as the number of viral particles per certain volume required to transduce a single cell. Functional titration methods permit detection of integrated proviral DNA, transgene mRNA or protein expression levels in target cells. Titration methods based on protein expression are typically based on vector-encoded reporter gene expression or staining with an antibody against the transgene. For instance, vectors expressing the fluorescent reporter gene (GFP, luciferase) can be titrated by fluorescence-activated cell sorting (FACS) analysis or immunofluorescence staining with specific antibodies against protein reporters. For vectors that do not contain a reporter gene, the titers can be determined by the integration of proviral or transgene copies into host cell genomic DNA using quantitative PCR (qPCR). To determine the integrated virus for each cell, standard curves must be prepared with DNA materials that contain a known amount of PCR targets. So far, qPCR-based methods have been recognized as the gold standard for lentivirus infection titers.
Risks of Imprecise Titration
| Risk Category | Consequences of Undercounting (Under-Titered Vector) | Consequences of Overcounting (Over-Titered Vector) |
|---|---|---|
| Experimental/Efficacy | Failure of therapeutic gene expression or target cell transduction can lead to false-negative research results or poor efficacy, wasting time and resources. | Overtransduction leading to nonspecific effects, cytotoxicity, or saturation of cellular machinery may mask the true biological effect (false-positive/confounded results). |
| Safety & Toxicity | The risk of acute toxicity is low, but higher doses and repeated administration may be required, increasing overall costs and patient burden. | The risk of acute and chronic toxicity is increased due to higher multiplicities of infection (MOI), including cytotoxicity of the VSV-G envelope and a higher statistical risk of insertional mutagenesis per treated subject. |
| Cost & Compliance | Inefficient use of expensive vector inventory can result in higher-than-expected doses. Regulators require precise dosing information in Investigational New Drug (IND) applications. Inaccurate data can lead to IND application hold or trial failure. | Waste of precious vector material. Over-reporting of titers requires using less product, but can lead to the efficacy risks mentioned above if the actual functional titer is much lower. |
Core Services at Creative Biolabs
Creative Biolabs is a leading commercial supplier of viral vector technologies with state-of-the-art technology and versatile platforms for LVs titration. We provide robust and strong titration methods based on your specific application, such as FACS analysis for your clinical studies and other gene therapy applications. Our robust and optimized processes will save you time and reduce variability in your research. In addition to the titer service, many other customizable options are already available at Creative Biolabs and we will continue to consider your requests to bring you more flexibility.
Customizable Options
- Lentiviral Vector Optimization Service
- Lentiviral Vector Design for Regulated Integration and Expression
- Safety Determination of Lentiviral Vector Service
- Advanced Lentiviral Vector Development Service for Basic Research
- Advanced Lentiviral Vector Development for Gene Therapy
Features
- The highly experienced team delivers high-quality LVs services
- Consistent and reliable titers delivered at or above specified titer levels
- Expert technical support, full confidentiality, and on-time delivery with all projects completed on-site
Customer Review

"The comprehensive carrier characterization service has revealed key insights into our lentiviral formulation, which standard single method titration may miss. Although our internal p24 measurements indicate sufficient carrier concentration, comprehensive physical and functional analysis suggests that our production method primarily produces noninfectious particles. This discovery prompted process optimization, increasing our functional titers by 5 times, saving months of troubleshooting and resource waste."
— Professor Katherine Li, Department of Genetic Medicine
Frequently Asked Questions
Q: How much viral sample is required for accurate titration?
A: For standard functional titration, we recommend providing ≥50 μL of concentrated lentiviral preparation (expected titer ≥1 × 10^7 TU/mL). For comprehensive physical and functional characterization, ≥100 μL is ideal, allowing for all analytical procedures and repeat testing as needed.
Q: How stable are lentiviral vectors during shipping and storage?
A: Lentiviral vectors are inherently unstable, and functional titers decline rapidly under suboptimal conditions. We explicitly recommend snap freezing in liquid nitrogen and storage/shipping at ≤ -80°C. Even under ideal conditions, functional titers typically decline significantly within a few weeks at -80°C, so immediate titration and use are recommended.
Q: Why do physical titration methods (p24, qPCR) typically yield higher values than functional titration methods?
A: This discrepancy reflects the fundamental differences in what each method measures. Physical methods quantify all viral particles containing the target component (protein or nucleic acid), including both infectious and non-infectious particles. Functional methods specifically measure particles capable of completing the transduction process. Therefore, the ratio of infectious particles to physical particles is an important quality indicator; a higher ratio (e.g., 1:10 to 1:100) generally indicates a higher-quality vector preparation.
Q: What MOI should I use for my specific cell type?
A: The optimal MOI is highly dependent on the cell type and must be determined empirically through preliminary ranging experiments. As a general guide, easily transducible cell lines (HEK293T, HeLa) typically achieve high transduction at an MOI of 1-5, while challenging primary cells (hematopoietic stem cells, neurons) may require an MOI of 20-100 or higher. We offer cell-specific MOI optimization as a specialized service to support critical applications.
Q: Can you help us solve the problem of low titer in internal LVV production?
A: Of course. Our team provides technical consultation to identify issues such as plasmid quality, transfection efficiency, and purification methods. We recently helped a client increase their LVV titer from 5 × 10 ^ 5 TU/mL to 3 × 10 ^ 7 TU/mL by optimizing the packaging plasmid ratio.
Q: How do you ensure the reproducibility of titration results?
A: We use validated protocols, reference standards (such as NIST traceable GFP standard), and inter batch controls. Our internal quality control program requires each sample to undergo 3 independent runs, and only accepts results when CV<10%.
Reach Out to Us Now!
Along with providing high-quality LVs titration service, Creative Biolabs offers its expertise and support to clients in areas such as LVs project management, scientific design, and project follow-up. If you have any question about our lentivirus services or don't see the service you're looking for, please feel free to contact us.
Reference
- Parr-Brownlie L C, Bosch-Bouju C, Schoderboeck L, et al. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Frontiers in molecular neuroscience, 2015, 8: 14. https://doi.org/10.3389/fnmol.2015.00014 (Distributed under Open Access license CC BY 4.0, without modification.)
