Group I Intron Self-Splicing System
Creative Biolabs is a world-class CRO and well-known expert in gene therapy. Our combination of proven circular RNA (circRNA) products and custom services ensures assistance to customers in the pharmaceutical and life science industries.
Introduction to CircRNAs
CircRNAs are novel and special RNAs characterized by the formation of covalently closed continuous loops. In general, circRNAs are abundant and stable in various species. Furthermore, circRNAs combine with RNA-associated proteins to form RNA-protein complexes, which play an indispensable role in regulating gene transcription and peptide formation. Numerous pieces of evidence have revealed that circRNAs have great potential in serving as diagnostic and predictive biomarkers in tumorigenesis, which provides a new direction for cancer diagnosis and targeted therapy. Therefore, the synthesis of circRNAs has attracted increasing attention.
Synthetic Strategies for CircRNAs
Several circRNAs biological synthesis methods have been developed. Among them, the enzymatic connection strategy and ribozyme strategy are the primary methods for in vitro production circRNAs. The first approach depends on the T4 RNA or T4 DNA ligase enzyme to induce RNA to circulate, but this method is not suitable for in vivo ligation. The latter method depends on self-splicing to induce RNA circularization. At present, this method has occupied an important position in the circRNAs ligation by the regular group I intron self-splicing reaction, containing two transesterifications at the specified splice site. This approach has a wide range of applications and is applicable to RNA circularization in vitro as well as in vivo.
The Mechanism of Group I Intron Splicing
This process relies on a two-step transesterification with exogenous guanosine-5'-triphosphate (αG). In the first process, αG is linked to the 5' end of the intronic RNA via a 3'-5' phosphodiester bond. The ensuing conformational change allows the terminal 3' guanosine (ωG) of the upstream exon to swap places with αG to mobilize the second transesterification reaction. The 3'-OH of the upstream exon attacks the 3' splice site, facilitating the junction of upstream and downstream exons and the release of intronic RNA.
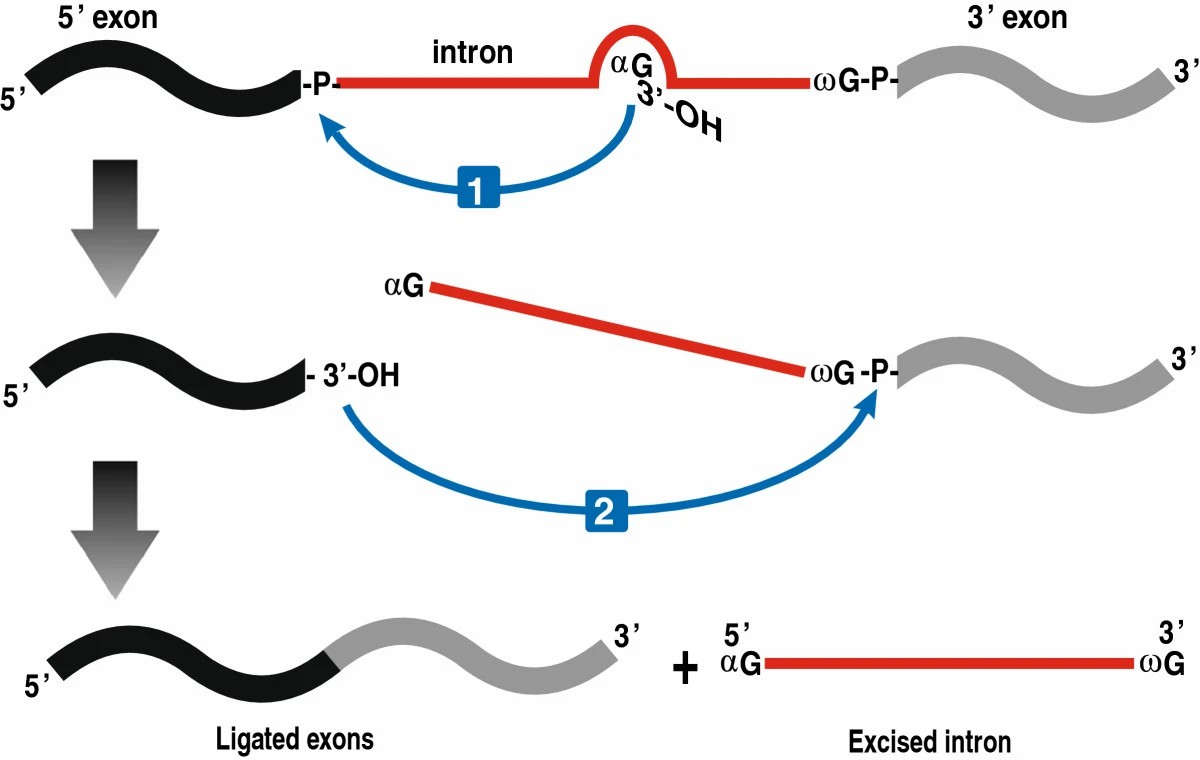 Fig.1 Splicing schematic.¹
Fig.1 Splicing schematic.¹
Advantages of Splicing
- It is suitable for the ligation of larger linear RNA precursors.
- The reaction conditions are simple.
- Purification is simple and convenient.
- This method allows for accurate RNA ligation.
Disadvantages of Splicing
- It is susceptible to the complexity of RNA secondary structure.
- The introduction of exon sequences may affect the final circRNA sequence.
Dedicated to being your best gene therapy research partner, Creative Biolabs specializes in providing comprehensive circRNAs products and services to our customers. In addition to our diverse services, we also accept special customization requests from our customers. If you have any difficulties in the gene therapy project, please contact us in time for professional advice.
Reference
- Hausner, Georg, Mohamed Hafez, and David R. Edgell. "Bacterial group I introns: mobile RNA catalysts." Mobile DNA 5.1 (2014): 1-12. Distributed under Open Access license CC BY 4.0, without modification.
