PTPRD and Associated Diseases
Researchers have conducted relatively in-depth neurobiological and genomic studies on the receptor-type protein tyrosine phosphatase D-type receptor (PTPRD), and determined its function in vivo and its correlation with various diseases.
Structure and Function of PTPRD
Cryo-electron microscopy reveals three key domains of PTPRD. The D1 phosphatase domain of PTPRD is proximal to the membrane and is responsible for phosphatase activity. Although the D2 domain is highly homologous to the phosphatase sequence in structure, it does not have sufficient enzymatic activity, but binds to the phosphatase domain of PTPRD/PTPRD-related proteins to inhibit its activity. PTPRD has a relatively low affinity for phosphotyrosine substrates but has good catalytic activity, thereby achieving dephosphorylation of phosphotyrosine residues in cells.
The extracellular structure of PTPRD is responsible for functional interactions with cognate PTPRD, SLITRK, IL1RAPL1 and LRRC4B proteins. The extramembrane immunoglobulin and fibronectin III domains of PTPRD ensure its role as a cell adhesion molecule and synapse specifier, supporting active neurotrophic and adhesive roles in neuronal subpopulations.
PTPRD in Cancer
As an important tumor suppressor gene, PTPRD is down-regulated by promoter hypermethylation in the pathogenesis of many tumors. The inactivation or disorder of PTPRD is related to many epigenetic and chromosomal abnormalities, including DNA methylation, gene amplification, chromosome deletion and rearrangement. PTPRD directly dephosphorylates STAT3, prevents its dimerization, and negatively regulates its downstream pathways. Microarray analysis also showed that the expression of CXCL8 was significantly increased after PTPRD loss, thereby inducing invasion, apoptosis, migration and immunosuppressive processes of cancer. In addition, immunohistochemical experiments and genomic analysis confirmed that PTPRD participates in several signaling pathways in the pathogenesis of many cancers, including PTPRD-PTPRD self-combination, β-catenin, TCF, JAK, PTPRD-PI3K-mTOR, etc.
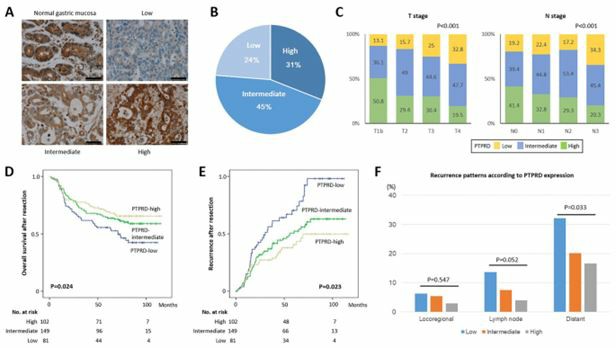 Fig 1. PTPRD expression and its significance in gastric cancer. (Bae, 2019)
Fig 1. PTPRD expression and its significance in gastric cancer. (Bae, 2019)
PTPRD in Other Diseases
In addition to its important role in the pathogenesis of tumors, PTPRD has also been shown to be associated with many non-tumor diseases. PTPRD haplotypes and variants up-regulate the contents of STING and IFN-α in dorsal root ganglia, and participate in the process of neuropathic pain. Administration of PTPRD inhibitors or direct knockout of PTPRD can effectively alleviate the development of pathological pain in rats. PTPRD has also been shown to be involved in drug addiction, and inhibition of PTPRD enhances the vulnerability exhibited during drug truncation. A strong and oligogenic relationship between PTPRD and restless legs syndrome has been confirmed by locus studies, especially the single nucleotides rs4626664 and rs1975197 located in the PTPRD intron. In addition, rs560380 on the PTPRD intron has a direct and clear association with neurofibrillary tangles, suggesting that variation in the expression level of PTPRD may generate neurofibrillary tangles and thus affect the pathogenesis of neurodegenerative diseases.
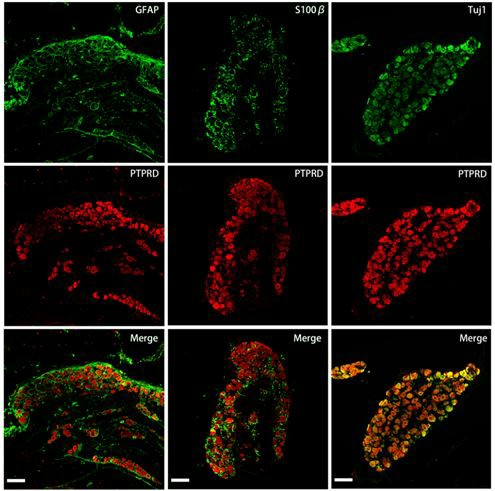 Fig 3. Chronic constriction injury induces PTPRD expression in dorsal root ganglion neurons. (Sun, 2022)
Fig 3. Chronic constriction injury induces PTPRD expression in dorsal root ganglion neurons. (Sun, 2022)
Related Services
Creative Biolabs provides the most comprehensive, stable and repeatable analysis and detection services for PTPRD and associated diseases to clients all over the world. Our services include but are not limited to gene editing, gene sequencing, protein analysis, and tissue sampling. In addition, we are also happy to communicate with you and optimize existing experimental procedures or develop new methods according to your specific needs. Please feel free to contact us.
References
- Bae, W.J.; et al. PTPRD-inactivation-induced CXCL8 promotes angiogenesis and metastasis in gastric cancer and is inhibited by metformin. Journal of Experimental and Clinical Cancer Research. 2019, 38: 484. Distributed under Open Access license CC BY 4.0, without modification.
- Sun, C.; et al. Protein tyrosine phosphatase receptor Type D regulates neuropathic pain after nerve injury via the STING-IFN-I pathway. Frontiers in Molecular Neuroscience. 2022, 15: 859166. Distributed under Open Access license CC BY 4.0, without modification.
