Introduction of GRIN3A
Glutamate receptor ionotropic, NMDA 3A (GRIN3A), is a subunit of the NMDARs, which are heterotetrameric combinations of an obligatory GRIN1 subunit, at least one GRIN2 (A-D), and in some cases GRIN3 (A, B) subunits. Different subtypes are differentially expressed during development and exhibit ionic conductance with distinct properties, amplitude, and duration. The GRIN3A subunit gives a remarkable modification of the NMDARs. The inclusion of GRIN3A subunit in NMDAR channels reduces their calcium permeability and sensitivity to magnesium blockade at hyperpolarized potential, thus promoting a reduction in NMDA-induced currents. In humans, GRIN3A is developmentally regulated and it peaks in the first years of life but is largely downregulated afterward. GRIN3A subunits are dependent on the co-assembly with GRIN1 subunits to be expressed at the surface membrane.
| Basic Information of GRIN3A | |
| Protein Name | Glutamate receptor ionotropic, NMDA 3A |
| Gene Name | GRIN3A, KIAA1973 |
| Aliases | NMDAR3A, NR3A |
| Organism | Homo sapiens (Human) |
| UniProt ID | Q8TCU5 |
| Transmembrane Times | 3 |
| Length (aa) | 1,115 |
| Sequence | MRRLSLWWLLSRVCLLLPPPCALVLAGVPSSSSHPQPCQILKRIGHAVRVGAVHLQPWTTAPRAASRAPDDSRAGAQRDEPEPGTRRSPAPSPGARWLGSTLHGRGPPGSRKPGEGARAEALWPRDALLFAVDNLNRVEGLLPYNLSLEVVMAIEAGLGDLPLLPFSSPSSPWSSDPFSFLQSVCHTVVVQGVSALLAFPQSQGEMMELDLVSLVLHIPVISIVRHEFPRESQNPLHLQLSLENSLSSDADVTVSILTMNNWYNFSLLLCQEDWNITDFLLLTQNNSKFHLGSIINITANLPSTQDLLSFLQIQLESIKNSTPTVVMFGCDMESIRRIFEITTQFGVMPPELRWVLGDSQNVEELRTEGLPLGLIAHGKTTQSVFEHYVQDAMELVARAVATATMIQPELALIPSTMNCMEVETTNLTSGQYLSRFLANTTFRGLSGSIRVKGSTIVSSENNFFIWNLQHDPMGKPMWTRLGSWQGGKIVMDYGIWPEQAQRHKTHFQHPSKLHLRVVTLIEHPFVFTREVDDEGLCPAGQLCLDPMTNDSSTLDSLFSSLHSSNDTVPIKFKKCCYGYCIDLLEKIAEDMNFDFDLYIVGDGKYGAWKNGHWTGLVGDLLRGTAHMAVTSFSINTARSQVIDFTSPFFSTSLGILVRTRDTAAPIGAFMWPLHWTMWLGIFVALHITAVFLTLYEWKSPFGLTPKGRNRSKVFSFSSALNICYALLFGRTVAIKPPKCWTGRFLMNLWAIFCMFCLSTYTANLAAVMVGEKIYEELSGIHDPKLHHPSQGFRFGTVRESSAEDYVRQSFPEMHEYMRRYNVPATPDGVEYLKNDPEKLDAFIMDKALLDYEVSIDADCKLLTVGKPFAIEGYGIGLPPNSPLTANISELISQYKSHGFMDMLHDKWYRVVPCGKRSFAVTETLQMGIKHFSGLFVLLCIGFGLSILTTIGEHIVYRLLLPRIKNKSKLQYWLHTSQRLHRAINTSFIEEKQQHFKTKRVEKRSNVGPRQLTVWNTSNLSHDNRRKYIFSDEEGQNQLGIRIHQDIPLPPRRRELPALRTTNGKADSLNVSRNSVMQELSELEKQIQVIRQELQLAVSRKTELEEYQRTSRTCES |
Functions of GRIN3A Membrane Protein
Studies have suggested that GRIN3A may influence the spine dynamics and may be involved in NMDAR hypofunction. Growing evidence in recent years has implicated GRIN3A in various disorders of the central nervous system (CNS). First of all, GRIN3A has been directly implicated in Huntington’s disease (HD) and in cocaine addiction. In mouse studies, motor and cognitive deficits, as well as decreases in spine density and striatal atrophy of medium spiny neurons (MSN), are rescued in HD mouse models lacking GRIN3A. Furthermore, there are additional reports confirming GRIN3A neuroprotective properties in ischemia and in striatal lesions. These studies have suggested prospects for development of agonists and antagonists targeting the GRIN3A-containing NMDARs to treat these brain disorders. Additionally, GRIN3A distribution outside the postsynaptic densities, including presynaptic astrocytes, places it at a strategic position to play an important role in the interactions between neurons and glial cells.
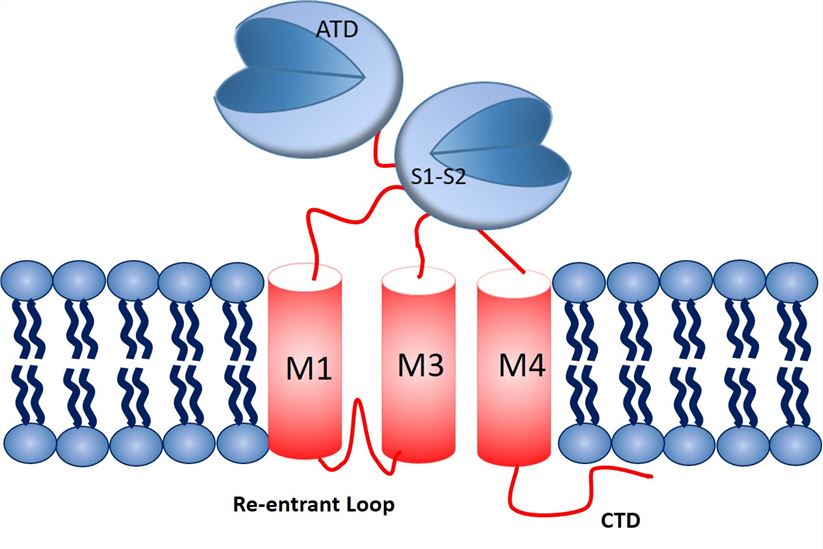 Fig.1 NMDA subunit structure and topology.
Fig.1 NMDA subunit structure and topology.
Application of GRIN3A Membrane Protein in Literature
Using confocal time-lapse imaging in rat hippocampal organotypic slices, this study tested the hypothesis that the GluN3A subunit of NMDARs played a role as a molecular brake in synaptic maturation and assessed the role of these GluN3A-containing NMDARs in spine dynamics. The results suggested GluN3A as a molecular signal for synapse maturation regulation.
This study investigated the role of GluN3A in Huntington’s disease (HD). The results showed that overexpressing of GluN3A in wild-type mice striatum mimicked the synapse loss in HD mouse models, while genetic deletion reversed the neuropathological changes in the YAC 128 HD mouse model.
Using littermate wild-type and GluN3A knockout mice, this article investigated whether the GluN3A subunit showed a protective action against ischemia-induced brain injury. The results suggested GluN3A was a noteworthy regulator in ischemia-induced excitotoxicity and brain injury.
This study co-expressed N-terminal domain (NTD) deleted GluN1 and GluN3 subunits in Xenopus oocytes, which generated GluN1/GluN3 receptors with a large decrease in the glycine-inducible Imax accompanied by a strongly impaired GluN1 antagonist-mediated potentiation. These results indicated that the GluN3 NTD was a crucial regulatory determinant of the function of GluN1/GluN3A receptor.
Using wild-type and GluN3A knockout mice, this study demonstrated that genetic deletion of this subunit affected multiple behavioral functions in adult animals, such as impaired locomotor activity, increased sensitivity to inflammatory pain, enhanced recognition and spatial learning and memory function.
GRIN3A Preparation Options
To help facilitate the basic understanding of the biology of the GRIN3A subunit aid in drug design, Creative Biolabs has established our powerful and versatile Magic™ Membrane Protein Platform, to obtain purified, stabilized, functional formats of this membrane protein. This can be achieved by using various strategies and approaches, such as different types of detergents coupled with lipids or substitutive tools like liposomes, nanodiscs, and polymers. Moreover, we can help with the discovery of anti-membrane protein antibodies using our leading-edge technologies. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-GRIN3A antibody development services.
Supported by our leading technologies and years of experience in the field of membrane protein research, Creative Biolabs is confident in providing first-class products and services to our clients. If you are interested in any of our services, please feel free to contact us for more information.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.