Introduction of NPY4R
Neuropeptide Y receptor 4 (NPY4R) is a protein that in humans is encoded by the NPY4R gene. It is also know as Pancreatic polypeptide receptor 1 and a member of the Neuropeptide Y receptor family. It also belongs to the G-protein-coupled receptor superfamily. NPY receptor family comprises four functional NPY receptor subtypes (Y1R, Y2R, Y4R Y5R), it exerts a variety of physiological processes in humans via four different receptor subtypes. NPY4R involve in the control of appetite and energy metabolism. It also responds to pancreatic polypeptide (PP).
| Basic Information of NPY4R | |
| Protein Name | Neuropeptide Y receptor type 4 |
| Gene Name | NPY4R |
| Aliases | Y4, PP1, PPYR1, NPY4R |
| Organism | Homo sapiens (Human) |
| UniProt ID | P50391 |
| Transmembrane Times | 7 |
| Length (aa) | 375 |
| Sequence | MNTSHLLALLLPKSPQGENRSKPLGTPYNFSEHCQDSVDVMVFIVTSYSIETVVGVLGNLCLMCVTVRQKEKANVTNLLIANLAFSDFLMCLLCQPLTAVYTIMDYWIFGETLCKMSAFIQCMSVTVSILSLVLVALERHQLIINPTGWKPSISQAYLGIVLIWVIACVLSLPFLANSILENVFHKNHSKALEFLADKVVCTESWPLAHHRTIYTTFLLLFQYCLPLGFILVCYARIYRRLQRQGRVFHKGTYSLRAGHMKQVNVVLVVMVVAFAVLWLPLHVFNSLEDWHHEAIPICHGNLIFLVCHLLAMASTCVNPFIYGFLNTNFKKEIKALVLTCQQSAPLEESEHLPLSTVHTEVSKGSLRLSGRSNPI |
Function of NPY4R Membrane Protein
The NPY4R is predominantly expressed in peripheral tissues such as the pancreas, colon, liver, heart, and intestine. Importantly, it responds to pancreatic polypeptide (PP). This gene is a strong candidate for body weight regulation because PP is the preferential agonist of the Y4 receptor and functions as a satiety signal via the Y4 receptors. There are four NPY-family receptors in humans and all of them are expressed in the brain, especially in the hypothalamic regions that are involved in the control of appetite and energy metabolism as well as in the periphery. Peripheral administration of PP decreases the hypothalamic expression of the potent hunger stimulants NPY, ghrelin and orexin, and increases anorexigenic urocortin in animal models. So NPY4R might play a significant role in obesity.
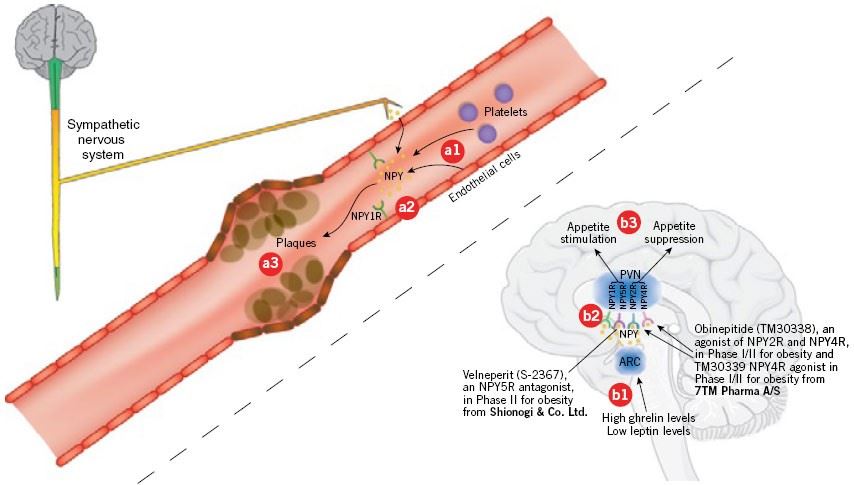 Fig.1 Neuropeptide Y in atherosclerosis and obesity. (Osherovich, 2009)
Fig.1 Neuropeptide Y in atherosclerosis and obesity. (Osherovich, 2009)
Application of NPY4R Membrane Protein in Literature
The authors hypothesized that both genetic and structural variation in NPY4R might be implicated in the pathogenesis of obesity. Their data support an essential role for genetic and structural variation within the NPY4R gene in the pathogenesis of obesity.
This article says the structure of PYY is proposed that potent binding to Y1, Y4, and Y5 receptors requires the juxtaposition of the two termini while Y2 binding only requires the C-terminal helix. And further experiments that delineate between primary and tertiary structure contributions for receptor binding and activation are required to support the hypothesis that tertiary structure is stable enough to influence the expression of PYY's bioactivity.
This article has investigated whether additional binding sites exist within the N-terminal domain, as studied in the form of binding of peptides from the neuropeptide Y (NPY) family to the N terminus of the Y4 receptor (N-Y4), and found the N-terminal domain of the Y4 receptor has been expressed in isotopically enriched form and studied by solution NMR spectroscopy.
This article aimed to investigate the molecular mechanism of hY4R desensitization and endocytosis and identified the internalization motif for the human NPY4R, which regulates arrestin-3 recruitment and receptor endocytosis.
This article identified PP1 as a novel 4.1N-interacting molecule, and the FERM domain of 4.1N mediated the interaction between 4.1N and PP1. Further, ectopic expression of 4.1N could inactivate the JNK-c-Jun signaling pathway through enhancing PP1 activity and interaction between PP1 and p-JNK.
NPY4R Preparation Options
To obtain the soluble and functional target protein, the versatile Magic™ membrane protein production platform in Creative Biolabs enables many flexible options, from which you can always find a better match for your particular project. Aided by our versatile Magic™ anti-membrane protein antibody discovery platform, we also provide customized anti-NPY4R antibody development services.
As a forward-looking research institute as well as a leading customer service provider in the field of membrane protein, Creative Biolabs has won good reputation among our worldwide customers for successfully accomplishing numerous challenging projects including generation of many functional membrane proteins. Please feel free to contact us for more information.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.