Introduction to Breast Cancer Spheroids
Three-dimensional (3D) cell culture models, particularly spheroids, represent a significant advancement in cancer research, gradually superseding conventional two-dimensional (2D) monolayer cultures. Unlike flat 2D cultures, breast cancer spheroids are self-assembling, spherical aggregates of cells that mimic crucial aspects of in vivo tumor architecture and physiology. This includes the establishment of cell-to-cell contacts, cell-extracellular matrix (ECM) interactions, and the formation of gradients in oxygen, nutrients, and waste products, which are characteristic of solid tumors. These features are critical for accurately studying tumor growth, metastasis, drug resistance, and the tumor microenvironment (TME). The development of various methods, such as spontaneous cell aggregation, low cell attachment supports, and scaffold-based technologies, has facilitated the widespread adoption of spheroid models in cancer research.
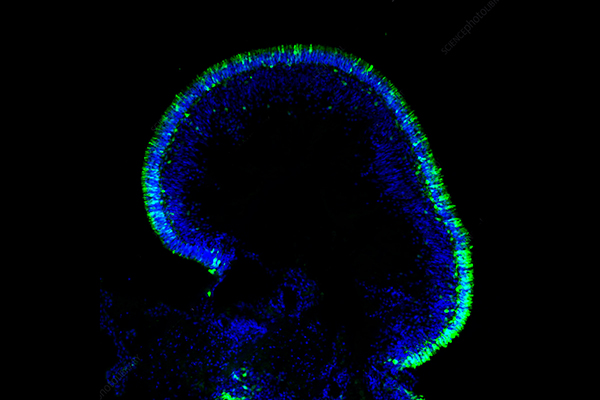
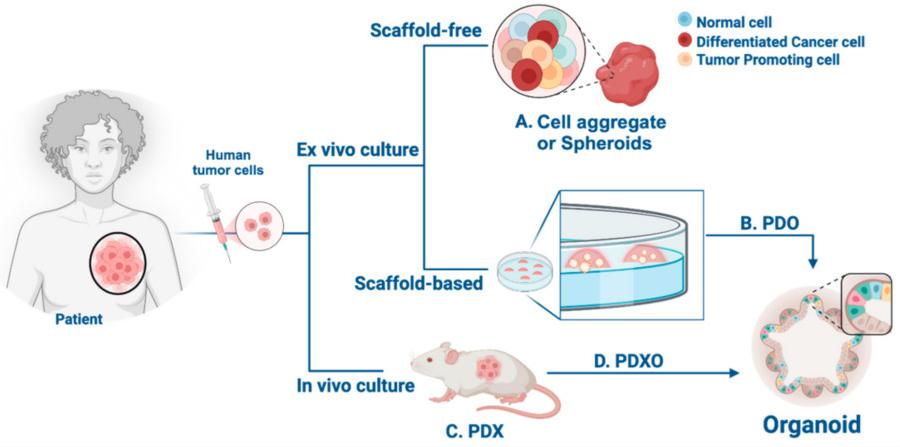 Figure 1 Schematic representation of epithelial tissue and novel 3D cell culture models for studying human tumor cells.1,3
Figure 1 Schematic representation of epithelial tissue and novel 3D cell culture models for studying human tumor cells.1,3
What is a Breast Cancer Stem Cell Spheroid?
Breast cancer stem cells (BCSCs) are a subpopulation of cancer cells characterized by their self-renewal capacity, ability to initiate new tumors, and resistance to conventional therapies. They are believed to be responsible for tumor initiation, progression, metastasis, and recurrence. A breast cancer stem cell spheroid, often referred to as a "mammosphere" when derived from mammary epithelial cells, is a 3D aggregate enriched in these highly tumorigenic and drug-resistant BCSCs.
These spheroids possess distinct characteristics that closely resemble the in vivo tumor niche:
-
Heterogeneous Cell Populations: Similar to solid tumors, spheroids can develop concentric zones, including an outer layer of highly proliferating cells, a middle layer of quiescent cells, and a necrotic core in larger spheroids due to oxygen and nutrient gradients. This cellular heterogeneity is crucial for mimicking tumor complexity.
-
Enhanced Cell-Cell and Cell-ECM Interactions: The 3D architecture promotes extensive cell-cell contacts and the deposition of endogenous ECM, which collectively influence cell behavior, signaling pathways, and drug response.
-
Stem-like Properties: Cells within these spheroids often exhibit increased expression of cancer stem cell markers (e.g., ALDH1, CD44+/CD24-), enhanced self-renewal capacity, and elevated drug resistance compared to their 2D counterparts. This makes them invaluable for studying drug target and developing targeted therapies.
Cell Sources of Breast Cancer Spheroids
Breast cancer spheroids can be generated from various cell sources, allowing for diverse research applications and the recapitulation of different aspects of breast cancer biology. The choice of cell source depends on the specific research question, ranging from established cell lines for high-throughput screening to patient-derived cells for personalized medicine approaches.
|
Cell Source Category
|
Specific Examples/Description
|
Key Advantages
|
|
Cancer Cell Lines
|
MDA-MB-231, MCF-7, T47D, SK-BR-3, BT-474
|
Easy to culture, highly reproducible, widely available, suitable for high-throughput drug screening. Represent different breast cancer subtypes (e.g., triple-negative, luminal, HER2+).
|
|
Primary Tumor Cells
|
Cells directly isolated from patient tumor biopsies
|
Best mimic of in vivo tumor heterogeneity and patient-specific characteristics, valuable for personalized medicine and drug toxicity testing.
|
|
Cancer Stem Cells (CSCs)
|
Isolated via markers (e.g., ALDH+, CD44+/CD24-) or functional assays (spheroid formation)
|
Enriched for highly tumorigenic and drug-resistant populations, ideal for studying recurrence and resistance mechanisms.
|
|
Co-culture Models
|
Breast cancer cells co-cultured with stromal cells (e.g., fibroblasts, endothelial cells, immune cells), adipose-derived stromal cells (hASCs)
|
Reconstitute the tumor microenvironment (TME), enabling study of complex cell-cell interactions, ECM remodeling, and their impact on tumor behavior and drug response.
|
Spheroids in Breast Cancer ALDH Drug Testing
Aldehyde Dehydrogenase 1 (ALDH1) activity is widely recognized as a functional marker for cancer stem cells (CSCs), including BCSCs, and is associated with tumor initiation, progression, and therapy resistance. The ALDH family of enzymes detoxifies reactive aldehydes and plays a crucial role in cellular differentiation and oxidative stress mitigation. Consequently, ALDH-positive cells often exhibit increased survival and drug resistance.
Breast cancer spheroids, particularly those enriched in BCSCs, serve as an excellent model for evaluating novel therapeutics targeting ALDH1-positive populations.
-
Mechanism of Resistance: High ALDH activity contributes to drug resistance by detoxifying cytotoxic agents or influencing signaling pathways related to stemness and survival. For instance, studies indicate that ALDH1A3 is a promising therapeutic target in aggressive breast cancers.
-
Oncology Drug Discovery: Researchers utilize spheroids to screen compounds that inhibit ALDH activity, thereby sensitizing ALDH-positive BCSCs to conventional chemotherapy. Inhibition of ALDH in combination with standard therapies is a promising strategy to overcome resistance in high ALDH-expressing tumors.
-
Predictive Value: Drug testing on spheroids with varying ALDH expression profiles can provide more accurate predictions of clinical response, as these models better reflect the heterogeneous drug penetration and cellular responses observed in solid tumors.
Breast Cancer Spheroid Invasion Collagen Assay
Tumor invasion into the surrounding extracellular matrix (ECM) is a critical step in metastasis, a major cause of mortality in breast cancer patients. The collagen invasion assay using breast cancer spheroids provides a robust and physiologically relevant in vitro model to study this complex process. Collagen type I is a major component of the interstitial matrix in solid tumors, making it an ideal substrate for invasion studies.
Methodology
-
Spheroid Formation: Breast cancer cells are cultured to form uniform spheroids using methods like hanging drops or ultra-low attachment plates.
-
Collagen Matrix Preparation: A neutralized collagen type I solution is prepared and allowed to solidify in multi-well plates, forming a 3D gel.
-
Spheroid Embedding: Individual spheroids are carefully embedded within or on top of the pre-formed collagen matrix. This allows the cells to interact with and invade the 3D environment.
-
Invasion Monitoring: The invading cells are monitored over time using bright-field or fluorescence microscopy. Quantitative analysis can be performed by:
-
Measuring the total area of invasion (e.g., using image analysis software).
-
Counting the number of invasive cells extending from the spheroid.
-
Assessing changes in cell morphology and F-actin organization within the matrix.
Drug/Treatment Evaluation
This assay is highly valuable for evaluating the anti-invasive potential of novel drugs or therapeutic interventions. For example, researchers can assess how a treatment impacts the ability of breast cancer spheroids to degrade and migrate through the collagen matrix, providing insights into potential anti-metastatic effects. The assay is versatile and can be combined with fluorescent probes to study specific cellular processes during invasion.
Types of Breast Cancer Spheroids
Beyond their derivation method, breast cancer spheroids can be classified based on their cellular composition and the specific breast cancer subtype they represent, enabling targeted research.
|
Spheroid Type
|
Description
|
Relevant Breast Cancer Subtypes/Application
|
|
Monoculture Spheroids
|
Formed from a single type of breast cancer cell line (e.g., MCF-7, MDA-MB-231).
|
Ideal for studying intrinsic cancer cell biology, signaling pathways, and initial drug screening for specific breast cancer subtypes (Luminal A, Luminal B, HER2-enriched, Triple-Negative).
|
|
Heterotypic Spheroids (Co-culture)
|
Composed of breast cancer cells co-cultured with other cell types from the tumor microenvironment, such as cancer-associated fibroblasts (CAFs), endothelial cells, immune cells (e.g., macrophages, T-cells), or mesenchymal stromal cells (MSCs).
|
Crucial for modeling the complex interactions within the TME, studying angiogenesis, immune evasion, drug resistance mediated by stromal cells, and developing TME-targeted therapies. Example: MDA-MB-231 co-cultured with human adipose-derived stromal cells (hASCs) to study ECM deposition and drug penetration.
|
|
Patient-Derived Spheroids/Organoids (PDOs)
|
Derived directly from patient tumor tissue, maintaining the genetic and histological characteristics of the original tumor. More complex organoids replicate tumor architecture and function.
|
Most clinically relevant models for personalized medicine, predicting individual patient response to therapies, and validating biomarkers. They retain patient-specific traits and mimic in vivo heterogeneity.
|
|
Stem Cell-Enriched Spheroids
|
Spheroids specifically grown under conditions that enrich for cancer stem cell populations (e.g., non-adherent conditions with specific growth factors).
|
Used to study the biology of cancer stem cells, their role in recurrence and metastasis, and to screen drugs specifically targeting these resistant populations.
|
Applications of Breast Cancer Spheroids
Breast cancer spheroids have a wide range of applications in biomedical research and drug development. In basic research, they are used to study the fundamental mechanisms of breast cancer initiation, progression, and metastasis. For example, researchers can investigate how genetic mutations affect the formation and behavior of breast cancer spheroids. In drug development, they serve as an excellent pre-clinical model for high-throughput drug screening. Pharmaceutical companies can test the efficacy and toxicity of novel drugs on breast cancer spheroids before proceeding to more expensive in vivo animal studies. Additionally, breast cancer spheroids are valuable in personalized medicine, where patient-derived spheroids can be used to predict the response of an individual patient to different treatment modalities. This can help clinicians tailor treatment plans to maximize the effectiveness of therapy and minimize side effects.
Frequently Asked Questions
Q: How long does it take to form breast cancer spheroids?
A: The time required for spheroid formation can vary depending on the cell type and culture conditions. Generally, it can take anywhere from a few days to a couple of weeks. For example, some breast cancer cell lines may form small spheroids within 3 - 5 days, while primary breast cancer cells may take longer, up to 1-2 weeks.
Q: Can breast cancer spheroids be cryopreserved?
A: Yes, breast cancer spheroids can be cryopreserved. Specialized cryopreservation protocols, including the use of cryoprotectants, are employed to ensure the viability of the spheroids upon thawing. However, the success of cryopreservation may depend on the type of spheroid and the specific cell population within it.
Q: Are breast cancer spheroids suitable for all types of breast cancer research?
A: While breast cancer spheroids are highly valuable for many aspects of breast cancer research, they may not be suitable for every type of study. For example, studies that require a large - scale analysis of gene expression in a highly controlled environment may face some challenges due to the inherent heterogeneity of spheroids. However, with appropriate experimental design and analysis methods, their use can be optimized for a wide range of research questions.
What Creative Biolabs Can Provide
At Creative Biolabs, we are committed to advancing breast cancer research and drug discovery by providing state-of-the-art 3D cell culture services and resources. Our extensive expertise in breast cancer spheroid models ensures that researchers have access to highly reliable and physiologically relevant platforms for their studies.
Custom Services
-
Custom 3D Spheroid Generation: Produces reproducible 3D spheroids from diverse cell types, including cancer lines and patient-derived cells, customized for specific research needs.
-
Co-culture and Complex Spheroid Models: Create and analyze co-culture spheroids with varied cell types to accurately simulate specific tissue microenvironments.
-
3D Spheroid Based Biomarker Discovery: Services for generating and analyzing PDTS, offering a robust platform for drug response evaluation.
-
3D Spheroid Based Drug Discovery: Tumor spheroids are favored for drug screening as they effectively replicate in vivo drug penetration barriers and resistance mechanisms.
-
3D Spheroid Based Toxicity Evaluation: Spheroids serve as a more reliable platform for evaluating drug-induced toxicity, yielding better predictions of in vivo adverse effects than 2D models.
Culture Products
3D Spheroid Model
Creative Biolabs empowers researchers to overcome the limitations of 2D models, accelerating the discovery and development of more effective therapies for breast cancer. Contact us today to learn more!
Reference
-
Pinto B, Henriques A C, Silva P M A, et al. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics, 2020, 12(12): 1186. https://doi.org/10.3390/cancers16101859. Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Schematic representation of epithelial tissue and novel 3D cell culture models for studying human tumor cells.1,3
Figure 1 Schematic representation of epithelial tissue and novel 3D cell culture models for studying human tumor cells.1,3