Single domain antibodies (sdAbs) have proved to be excellent candidates for imaging of checkpoint molecules within the tumor microenvironment. The pharmacokinetic properties of sdAb-based imaging agents, as well as the nature of the epitopes they recognize, may be useful attributes to capture the dynamics of the checkpoint molecules and the anti-tumor response under immunotherapy. Armed with rich experience in sdAb development and immune checkpoints research, Creative Biolabs provides customized services or products concerning noninvasive imaging of the immune checkpoint using sdAbs, from development to pre-clinical use.
Why Targeting Checkpoint Molecules with sdAbs?
Immune checkpoint inhibition is a promising cancer therapy, which has progressed rapidly from a preclinical concept to clinical implementation. Commonly considered checkpoint molecules are CTLA-4, PD-1/PD-L1, and LAG-3, and the list grows. Immune checkpoint blockade revolutionized anti-cancer therapy but unfortunately, only a subset of patients can benefit from it. The development of innovative and efficacious diagnostic methods is warranted. Thus, there is growing interest to do this noninvasively, by molecular imaging with target-specific tracers. SdAbs are small antigen-binding moieties that efficiently penetrate cell-cell interfaces in tumors and generate high contrast in noninvasive imaging, making them prime candidates for the development of novel imaging tracers.
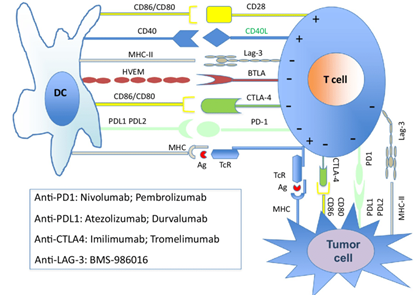 Fig.1 Illustration of the immune checkpoint molecules and their inhibitors.1,5
Fig.1 Illustration of the immune checkpoint molecules and their inhibitors.1,5
Popular Targets
PD-L1
Whole-body non-invasive imaging of PD-L1, which can provide visualization, localization and quantification of its expression throughout the body, is likely to be more informative and holds better prognostic value compared with immunohistochemistry (IHC). An anti-mouse PD-L1 sdAb (B3) was developed and labeled site-specifically with 18F. PET using the 18F-labeled anti-PD-L1 sdAb readily detected the B16 melanoma. An anti-human PD-L1 sdAb (sdAb K2) was also developed and used to non-invasively image mice bearing a tumor xenograft. The images showed high signal-to-noise ratios in tumors and detected PD-L1 in melanoma and breast tumors.
CTLA-4
In a previous study, a radiolabeled anti-CTLA-4 sdAb (H11) was used to non-invasively image CTLA-4 expression in a B16 melanoma model treated with anti-CTLA-4 antibody by PET. Scientists finally found that accessible CTLA-4 is largely confined to the tumor.
LAG3
LAG-3 is an inhibitory checkpoint molecule on T cells. Given the importance of LAG-3, anti-LAG-3 sdAbs have been developed and used for non-invasive imaging. The 99mTc-labeled sdAbs showed specific uptake in secondary lymphoid organs, and no signal was detected in LAG-3 knockout mice. LAG-3 expression was confirmed by flow cytometry and immunohistochemistry analyses and correlated well with the SPECT images.
| Imaging Field | Target | sdAb | Radiolabel | Related Disease | Notes |
| Checkpoint Molecules Imaging | Mouse PD-L1 | B3 | 18F | B16 melanoma | Ready to Use Nano-Imaging Tracer Products |
| Human PD-L1 | sdAb K2 | 99mTc | Melanoma, breast tumor, non-small cell lung cancer | ||
| CTLA-4 | H11 | 18F, 89Zr | B16 melanoma | ||
| LAG-3 | Nb 3132 | 99mTc | Myeloma |
Customized Services
- Generation and in vitro characterization of sdAb
- Nanotracers construction services
- In vitro assessment services
- In vivo imaging services
- Statistical analysis
As shown by the multitude of different sdAb-based imaging tools with promising pre-clinical effects reported in the last years, sdAb rises as very potent imaging agents. If you are interested in customized imaging services or products development, please feel free to contact us for more information.
Published Data
1. PD-L1-Targeting Single-Domain Antibody for Nuclear Imaging and Therapy
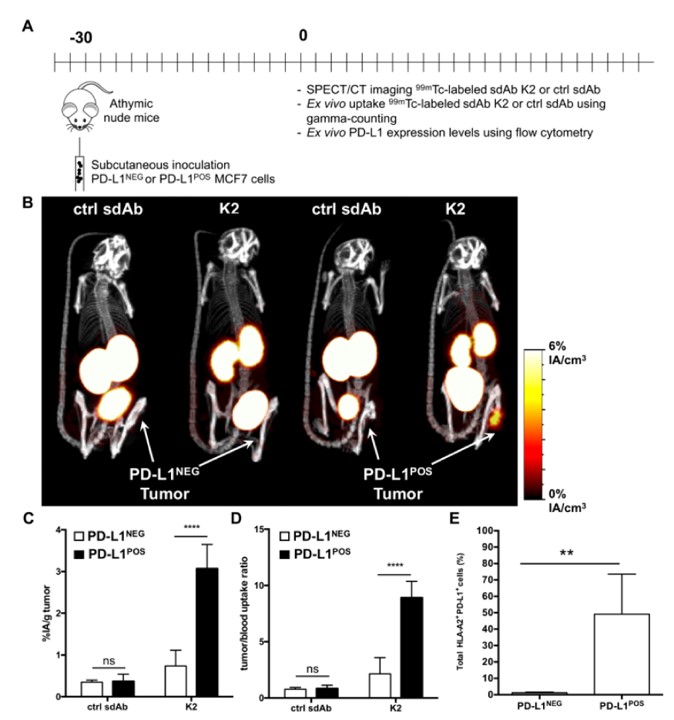 Fig.2 Nuclear imaging of human PD-L1POS breast tumors using radiolabeled sdAb.3,5
Fig.2 Nuclear imaging of human PD-L1POS breast tumors using radiolabeled sdAb.3,5
In this work, researchers developed human PD-L1-binding sdAbs and selected the high-affinity anti-PD-L1 sdAb. SPECT/CT imaging in mice, after intravenous administration of 99mTc-labeled sdAb, demonstrated strong tumor-specific detection in melanoma and breast cancer with high signal-to-noise ratios and minimal retention in the kidneys, a distinctive advantage of using radiolabeled sdAbs. Surface plasmon resonance revealed that sdAb binds to the same PD-L1 epitope as anti-PD-L1 monoclonal antibodies and effectively blocks PD-1:PD-L1 interactions. In cell-based assays, sdAb strengthened T-cell receptor signaling and tumor cell killing upon the interaction between PD-1POS T cells and PD-L1POS tumor cells. These findings support that anti-PD-L1 sdAb holds potential as a valuable diagnostic and therapeutic tool in cancer treatment.
2. Development and Preclinical Evaluation of Nanobody-Based Noninvasive Imaging of the Immune Checkpoint LAG-3
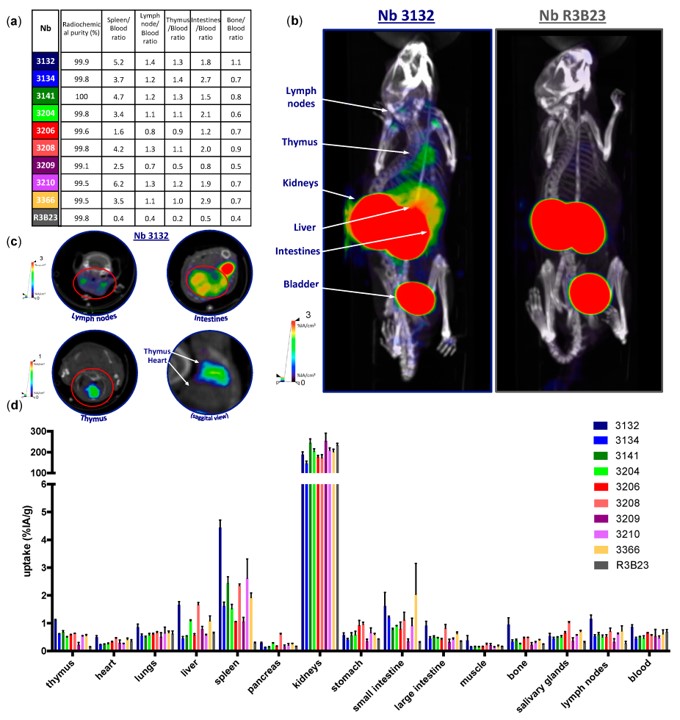 Fig.3 Imaging of mouse LAG-3 in naive C57BL/6 mice.4,5
Fig.3 Imaging of mouse LAG-3 in naive C57BL/6 mice.4,5
In this proof-of-concept study, researchers developed nanobodies, small fragments from camelid heavy chain-only antibodies, to noninvasively evaluate LAG-3 expression in mice using SPECT/CT imaging. Screening 114 nanobodies, nine were selected for binding to mouse LAG-3. Injection of 99mTc-labeled nanobodies into healthy mice showed specific uptake in immune organs, absent in LAG-3 knock-out mice. Additionally, the uptake of nanobodies was visualized using SPECT/CT imaging, and this data was correlated with the detection of LAG-3, which was evaluated through flow cytometry and immunohistochemical analysis. Tumor-bearing mice by SPECT/CT scans further validated the diagnostic potential of these nanobodies, supporting their use for imaging immune checkpoints in the tumor microenvironment.
References
- Rossi, Jean-François. "Immune precision medicine in cancer." Leukemia Research 85 (2019): S14.
- Ionescu, Sinziana. "The Use of Indocyanine Green in Colorectal Surgery." (2021).
- Broos, Katrijn, et al. "Evaluating a single domain antibody targeting human PD-L1 as a nuclear imaging and therapeutic agent." Cancers 11.6 (2019): 872.
- Lecocq, Quentin, et al. "Noninvasive imaging of the immune checkpoint LAG-3 using nanobodies, from development to pre-clinical use." Biomolecules 9.10 (2019): 548.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.