The presence or absence of particular biomarkers in the tumor extracellular matrix (ECM) may inform the choice of optimal treatment options. For scientists engaged in visualizing biological processes in a living animal, imaging the tumor ECM components by single domain antibody (sdAb) has always attracted wide attention. Creative Biolabs has been at the forefront of sdAb antibody research for many years. With the development of non-invasive imaging techniques, we provide sdAb-based tracer (Nanotracer) development services targeting various ECM components for imaging use. Besides customized development services, a series of ready-to-use catalogue is also available for you.
Why Targeting ECM?
ECM biomarkers selectively expressed at disease sites are attractive targets for imaging approaches. ECM deposition and modification are hallmarks of cancer and cancer cells make close contact with the ECM. The tumor ECM plays a role in the survival and progression of cancer cells, provides structural support and may affect the tumor response to treatment. Scientists need to better understand how the ECM responds to various treatments, and how it affects the dynamics and the interplay between cancer cells and the immune infiltrating cells. Non-invasive imaging of the ECM components and how they change in response to treatment may help researchers to better understand the effect of the ECM on treatment outcome. SdAbs against ECM biomarkers would be pertinent vehicles for the accumulation of imaging cargo at disease sites, potentially increasing specificity and reducing background.
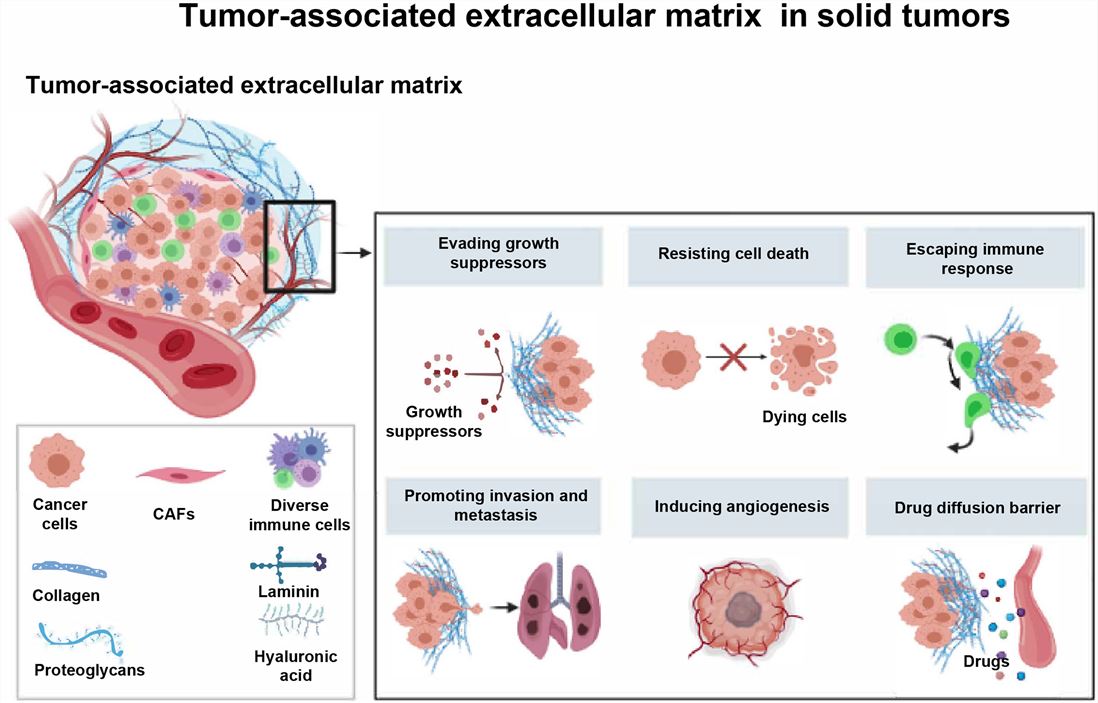 Fig.1 Schematic diagram of ECM.1,4
Fig.1 Schematic diagram of ECM.1,4
Imaging the ECM of Tumors
The ECM could be exploited to image cancer. For instance, a sdAb was developed that recognizes an alternatively spliced domain of fibronectin (fibronectin EIIIB) which is expressed in the ECM of a wide range of cancers, including pancreatic, melanoma, and breast cancer. The EIIIB-specific sdAb, VHH-NJB2, was radiolabeled and used to image different syngeneic and xenogeneic cancer models with primary tumors and metastatic lesions. The tumor ECM presents an interesting target for further exploration in the diagnosis of metastatic lesions.
| Imaging Field | Target | sdAb | Radiolabel | Related Disease | Notes |
| Tumor ECM Imaging | Fibronectin EIIIB | NJB2 | 64Cu | Triple-negative breast cancer, Pancreatic intraepithelial neoplasias; Pulmonary metastases | Ready to Use Nano-Imaging Tracer Products |
Customized Services
- Generation and in vitro characterization of sdAbs
- Nanotracers construction services
- In vitro assessment services
- In vivo imaging services
- Statistical analysis
Creative Biolabs provides novel strategies for delivering imaging probes specifically to the ECM in disease sites. If you are interested in our Nanotracer tool development and imaging services, please directly contact us and consult our technical supports online.
Published Data
1. Development of Nanobodies Targeting ECM Protein for Primary Tumors and Metastases Identification
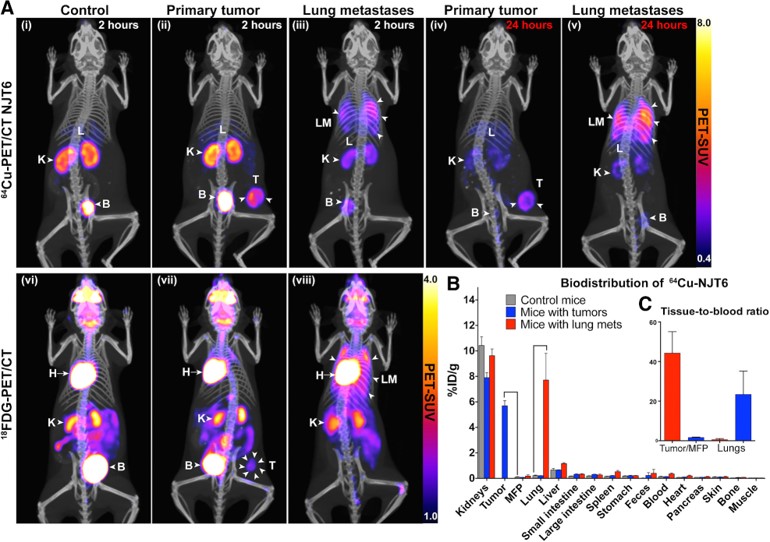 Fig.2 Noninvasive detection of tumors and lung metastases by immuno-PET/CT using 64Cu-labeled anti-TNC nanobodies.2,4
Fig.2 Noninvasive detection of tumors and lung metastases by immuno-PET/CT using 64Cu-labeled anti-TNC nanobodies.2,4
In this study, researchers developed libraries of nanobodies targeting ECM proteins expressed in human metastases, utilizing ECM-enriched extracts from colorectal cancer metastases and triple-negative breast cancer (TNBC) as immunogens. LC-MS/MS-based proteomics identified a 67-protein ECM signature shared by colorectal cancer metastases and TNBC. To demonstrate the potential of these libraries, the team isolated high-affinity nanobodies against tenascin-C (TNC), a protein in the ECM signature that is commonly expressed in primary tumors and metastases but rarely in normal adult tissues. Immuno-PET/CT imaging demonstrated that anti-TNC nanobodies selectively bind to metastases and TNBC tumors with high specificity. These nanobodies showed excellent specificity and clarity in vivo PET/CT imaging, successfully identifying both primary tumors and lung metastases, demonstrating their potential for diagnostic imaging in cancer.
2. Preclinical Development of a Fibroblast Activation Protein α-Targeted Radiotheranostic Single-Domain Antibody
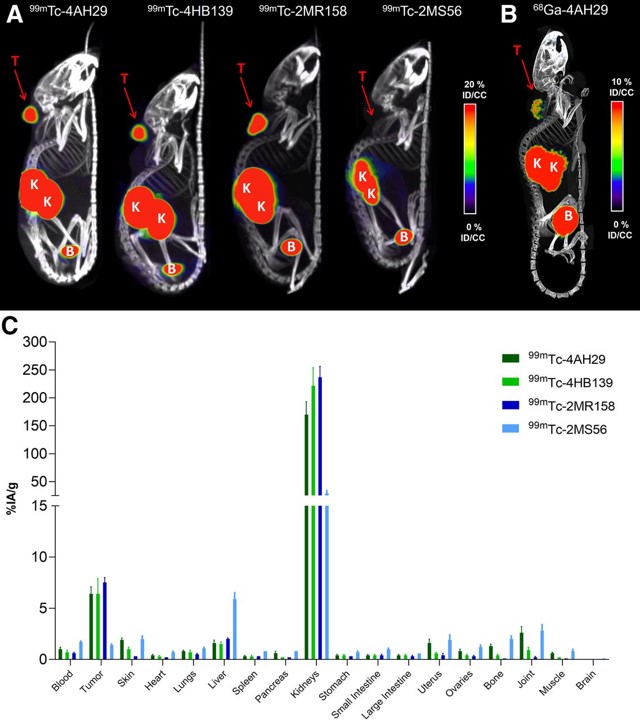 Fig.3 Biodistribution of 99mTc- and 68Ga-labeled anti-FAP sdAbs.3,4
Fig.3 Biodistribution of 99mTc- and 68Ga-labeled anti-FAP sdAbs.3,4
This study detailed the development of a sdAb targeting fibroblast activation protein (FAP) and evaluated its imaging and therapeutic potential in mice. The sdAb was radiolabeled with 131I and 68Ga for SPECT and PET imaging, and with 131I and 225Ac for targeted radionuclide therapy. The sdAb demonstrated a picomolar affinity for a unique FAP epitope, recognizing both purified and membrane-associated FAP proteins. Radiolabeled versions, including [68Ga]Ga-DOTA-sdAb, [225Ac]Ac-DOTA-sdAb, and [131I]I-guanidinomethyl iodobenzoate (GMIB)-sdAb, showed high radiochemical purity (>95%) and specifically bound to FAP-positive human fibroblasts and recombinant human FAP protein. These radiolabeled compounds demonstrated fast and selective accumulation in FAP-positive U87-MG glioblastoma tumors, with minimal yet specific uptake in normal tissues. Mice receiving [225Ac]Ac-DOTA-4AH29 and [131I]I-GMIB-4AH29 treatments showed extended survival times compared to controls. The results highlight the potential of radiolabeled sdAbs as imaging and therapeutic agents for FAP-positive cancers, supporting clinical evaluation.
References
- He, Yingying, et al. "Tumor-associated extracellular matrix: how to be a potential aide to anti-tumor immunotherapy?" Frontiers in cell and developmental biology 9 (2021): 739161.
- Jailkhani, Noor, et al. "Proteomic profiling of extracellular matrix components from patient metastases identifies consistently elevated proteins for developing nanobodies that target primary tumors and metastases." Cancer Research 83.12 (2023): 2052-2065.
- Dekempeneer, Yana, et al. "Preclinical Evaluation of a Radiotheranostic Single-Domain Antibody Against Fibroblast Activation Protein α." Journal of Nuclear Medicine 64.12 (2023): 1941-1948.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.