In the past years, many research projects at Creative Biolabs were aimed to develop sdAb-tracers for imaging of membrane cancer biomarkers. Examples of successful projects include those targeting epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), hepatocyte growth factor (HGF) and carcinoembryonic antigen (CEA) in different tumor models. Creative Biolabs is currently expanding our services & products list of oncology tracers in collaboration with strategically-chosen academic and industrial partners. Due to their small size, high thermal and chemical stability, nanomolar-range affinities, high specificity, and fast blood clearance, sdAbs should preferentially be selected for tumor in vivo imaging. Besides ready-to-use catalogue, Creative Biolabs makes full advantages of different labels and provides customized sdAb-based tracers (Nanotracers) development services for in vivo cancer biomarkers imaging research.
Hot Research Targets
- EGFR
EGFR is overexpressed in a variety of human tumors such as non-small cell lung cancer, breast, gastric, colorectal, bladder, renal, pancreatic, and ovarian cancers. Studies reported that anti-EGFR sdAb were evaluated as tracers for imaging of EGFR-positive xenografted tumors in mice. 99mTc-labeled sdAbs were cleared very fast out of blood and non-target organs and accumulated in A431 EGFR-positive tumors but not in EGFR-negative R1M tumors with high tumor-to-organ ratios.
- CEA
CEA is a biomarker for multiple cancer types, including colorectal carcinoma and adenocarcinomas of the lung, breast, other gastrointestinal organs and the ovaries. It is the target of the clinically-approved imaging tracer CEA-Scan, a 99mTc-labeled Fab fragment. SdAbs have also been successfully used to image CEA-positive xenografted tumors. For instance, the CEA specific sdAb (CEA1) was radiolabeled with 99mTc. Biodistribution and SPECT imaging studies in mice bearing CEA-positive human colon adenocarcinoma (LS174T) tumor showed both hepatic and renal clearance of the radiolabeled sdAb, resulting in low blood radioactivity levels.
- HER2
HER2 is a biomarker for breast and other types of cancers. Anti-HER2 sdAbs have been generated and biochemically evaluated for strength and specificity of antigen recognition, stability, targeted epitope and internalization rate. Different labels such as 99mTc, 68Ga, 89Zr, IRDye800CW have been used for in vivo sdAb-based radiopharmaceuticals evaluation.
- HGF
Elevated levels of HGF and c-Met have been observed in most solid tumors and are associated with increased aggressiveness of tumors. The potential of sdAbs for PET imaging of HGF-expressing tumors was reported. Two sdAbs, 1E2 and 6E10, were each molecularly fused to albumin-binding units (Alb8), conjugated with Df-Bz-NCS chelator and radiolabeled with 89Zr.
| Imaging Field | Target | Antibody | Radiolabel | Related Disease | Notes |
| Tumor Cell Imaging | HER2 | 2Rs15d | 99mTc, 111In, 177Lu, 18F, 225Ac, 68Ga, 131I | Breast Cancer |
Ready to Use Nano-Imaging Tracer Products |
| 5F7 | 125I, 131I, 18F | Breast Cancer | |||
| EGFR | 7C12, 7D12 | 99mTc, 177Lu, 68Ga, 89Zr | Skin Cancer | ||
| D10 | 99mTc | Skin Cancer | |||
| PSMA | PSMA30 | 99mTc | Prostate Cancer | ||
| JVZ-007 | 111In | ||||
| CEA | CEA5 | 99mTc | Breast Cancer | ||
| CD20 | 9077, 9079 | 99mTc, 111In, 177Lu, 68Ga | Non-Hodgkin lymphoma | ||
| HGF | 1E2-Alb8, 6E10-Alb8 | 89Zr | Glioma |
Customized Services
- Generation and in vitro characterization of sdAb
- Nanotracers construction services
- In vitro assessment services
- In vivo imaging services
- Statistical analysis
If you are interested in our Nanotracer tool development and imaging services, please directly contact us and consult our technical supports online.
Published Data
1. A Radiolabeled Single-Domain Antibody Fragment Targeting CD38 for Monitoring Multiple Myeloma
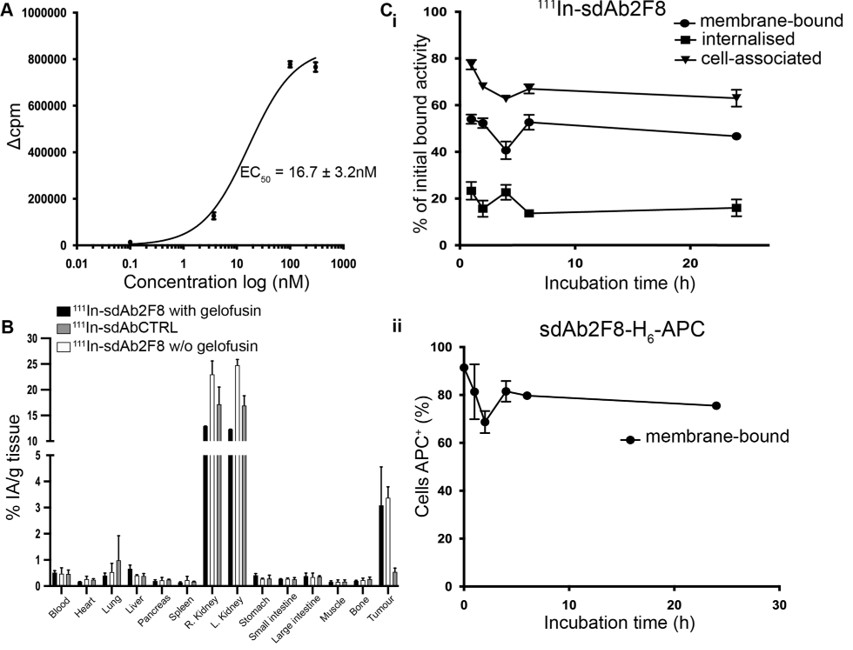 Fig.1 Binding kinetics and internalization of 111In-labelled sdAb.1
Fig.1 Binding kinetics and internalization of 111In-labelled sdAb.1
In this study, researchers identified a new anti-CD38 sdAb for tracing CD38+ tumor cells. Four anti-CD38 sdAbs were produced and tested for binding affinity and competition with an anti-CD38 monoclonal antibody (mAb) using biolayer interferometry. The binding kinetics and potential cell internalization were evaluated after radiolabelling with Indium-111. Results showed that the anti-CD38 sdAb demonstrated high affinity, stability, and no competition with the anti-CD38 mAb, with no receptor-mediated internalization. In vivo imaging with SPECT/CT and biodistribution studies in tumor-xenografted mice showed that the radiolabelled sdAb specifically targeted tumors and sustained tumor retention with low accumulation in non-tumor tissues, except the kidneys, resulting in high tumor-to-normal tissue ratios. This highlights the theragnostic potential of radiolabelled anti-CD38 sdAbs for multiple myeloma monitoring.
2. Evaluation of the Repeatability and Tumor Targeting of [68Ga]Ga-HER2 Single-Domain Antibody PET/CT Imaging in Breast Carcinoma
![Tracer biodistribution and uptake of HER2-negative patients who were injected twice with [68Ga]Ga-NOTA-anti-HER2-sdAb.](images/14-4-7-3-1-imaging-cancer-biomarkers-with-sdabs-2.jpg) Fig.2 Tracer biodistribution and uptake in normal tissues.2
Fig.2 Tracer biodistribution and uptake in normal tissues.2
This phase II study included 20 patients with locally advanced or metastatic breast cancer for evaluation of the repeatability and safety of the [68Ga]Ga-NOTA-anti-HER2 sdAb. The imaging technique demonstrated a repeatability coefficient of 21.8%, confirming its reliability and safety for clinical use. Blood samples were collected for antidrug antibody (ADA) testing and liquid biopsies, while immunohistochemistry, in situ hybridization, and mass spectrometry were used to assess the correlation between HER2 status and PET uptake values. When compared to [18F]FDG PET/CT (the standard of care), [68Ga]Ga-NOTA-anti-HER2 revealed intralesional HER2 expression heterogeneity in 7 out of 16 patients, a characteristic not detected by [18F]FDG. This tracer demonstrated greater sensitivity and specificity in determining disease extent, with imaging possible 90 minutes post-injection. These results provide a strong basis for the continued clinical advancement of [68Ga]Ga-NOTA-anti-HER2 as a PET/CT imaging agent in breast cancer.
References
- Duray, Elodie, et al. "A non-internalised CD38-binding radiolabelled single-domain antibody fragment to monitor and treat multiple myeloma." Journal of hematology & oncology 14 (2021): 1-13. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.
- Gondry, Odrade, et al. "Phase II Trial Assessing the Repeatability and Tumor Uptake of [68Ga] Ga-HER2 Single-Domain Antibody PET/CT in Patients with Breast Carcinoma." Journal of Nuclear Medicine 65.2 (2024): 178-184. Distributed under Open Access license CC BY 4.0, without modification
For Research Use Only.