Creative Biolabs is a leading service provider that focuses on polyclonal, monoclonal, and recombinant antibody development for research, diagnosis, and potential therapeutics. Especially, we have launched a series of in vitro diagnostic (IVD) antibody/immunoassay development services targeting different disease biomarkers. Here, we focus on the fecal S100A12 as a promising marker for both diagnosis and disease activity in the inflammatory bowel disease (IBD).
Introduction to S100A12
S100A12, also known as calgranulin C, is a member of the S100 family of proteins. In humans, it consists of twenty-five EF-hand (α helix-loop-α helix), calcium-binding proteins. It is mainly derived from neutrophils and monocytes/macrophages although some epithelial cells and dendritic cells also express this protein. S100A12 plays an important role in the regulation of inflammatory processes and immune response. By binding to the receptor for advanced glycation endproducts (RAGE), S100A12 activates the MAP-kinase and NF-kappa-B signaling pathways leading to the production of proinflammatory cytokines, chemotaxis, and increased oxidative stress.
![]()
Fecal S100A12 as A Diagnostic Marker of IBD
S100A12 has been suggested as a suitable marker of IBD, and studies have shown a correlation between mucosal inflammation and S100A12 levels in feces of patients with IBD. Researchers investigated the role of S100A12 in the prognosis of IBD and found that fecal levels of S100A12 were significantly elevated in the relapse group of patients than the non-relapse group, suggesting that S100A12 also has a potential role in predicting relapse. Besides, another research team reported fecal S100A12 as a non-invasive marker distinguishing IBD from irritable bowel syndrome (IBS) with high sensitivity and specificity. Moreover, S100A12 is remarkably resistant to degradation by fecal bacteria, and the stability of fecal specimens is acceptable for at least 7 days at room temperature. These studies have indicated that fecal S100A12 can be a potential non-invasive marker of IBD and diagnostic immunoassays can be developed targeting this marker and/or in multiplex with other markers to improve the diagnostic accuracy of IBD.
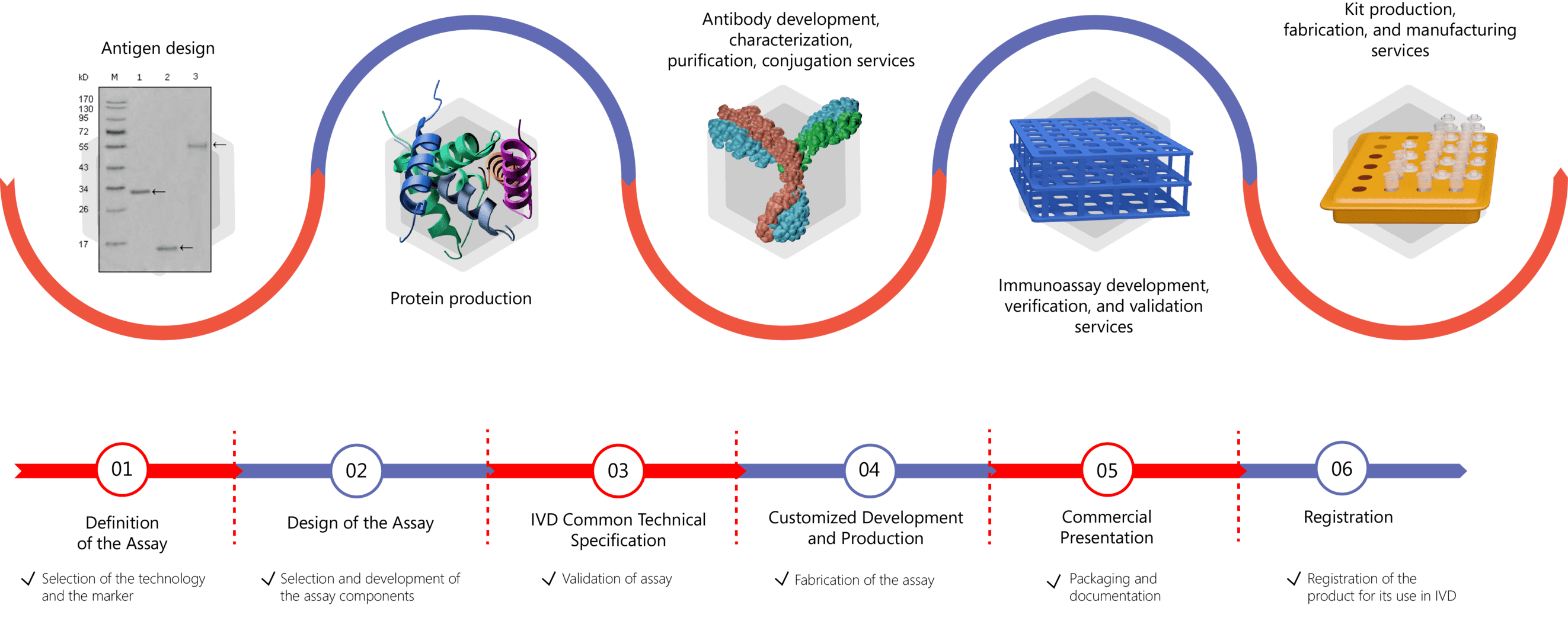
IVD Antibody/Immunoassay Development Services Provided by Creative Biolabs
Antibodies are core elements for immunoassays to detect and quantify analytes of interest in different samples such as the serum, urine, tissue preparations, and so on. At Creative Biolabs, we offer a full range of antibody development, antigen & antibody conjugation, and IVD immunoassay development services to clients across the globe. Our expertise lies in different stages of the immunoassay development process and different immunoassay technology platforms, including ELISAs, lateral flow immunochromatographic assays, immuno-PCR, etc. Creative Biolabs offers comprehensive contract development services including:
- IVD Antibody Development
- Antibody Pair Development
- Antibody & Protein Conjugation
- IVD Immunoassay Development
Features of Our Services
- Long-standing know-how and experience across a broad array of immunoassay technology platforms
- Strong scientific background and wide-ranging and multi-disciplinary expertise
- Flexibility and adaptability to deliver services designed to suit specific demands
- Efficient customer communication and regular project updates
Please feel free to contact us to discuss how our expertise can lead to your success.
For Research Use Only.