Creative Biolabs is a world-leading provider of custom aptamer selection and development services. We utilize our many years of experience and the latest cutting-edge technologies to deliver complete aptamer-based solutions to our international customer base for a wide range of cancer research, diagnostic and therapeutic applications.
Novel Solutions Driving Cancer Diagnostics
Early detection and accurate differentiation of cancer cells from normal cells are of utmost importance in cancer screening. The most aggressive cancers are often detected too late for successful treatment. Aptamers are short non-coding, single-stranded oligonucleotides developed through specific methods in vitro. Aptamers offer some unique abilities that are being applied to improve cancer detection. A variety of aptamers specifically bound to cancer biomarkers and cells had been selected. Moreover, taking advantage of nanomaterials, there were several aptamer-nanomaterial conjugates have been developed and widely investigated for diagnostics and targeted therapy of cancer.
Cancer Diagnosis Solutions
Selective aptamers for secreted tumor biomarkers and circulating tumor cells (CTCs) in acute myeloid leukemia, breast cancer, small cell lung cancer, and other forms of cancer are being widely used to develop more specific blood-based assays with enhanced sensitivity. Besides, selective aptamers are improving differentiation of tumor vs. normal tissue in MRI (magnetic resonance imaging), CT (computed tomography), and fluorescence imaging. Thus, a series of aptamers have been selected and applied in diagnostics.
- Detection of Cancer Biomarkers Using Aptamers
Biomarker detection plays a very important role in the early diagnosis and monitoring of curative effects. For instance, among the identified breast cancer-specific biomarkers, HER2 is one of the most important and commonly used biomarkers. It was reported that scientists once used cell-SELEX to select an anti-HER2 ssDNA aptamer which showed high binding affinity to HER2. Then, they developed a fluorescence-based system for HER2 in vitro detection and demonstrated its potential for the identification of the native HER2 protein in biological samples.
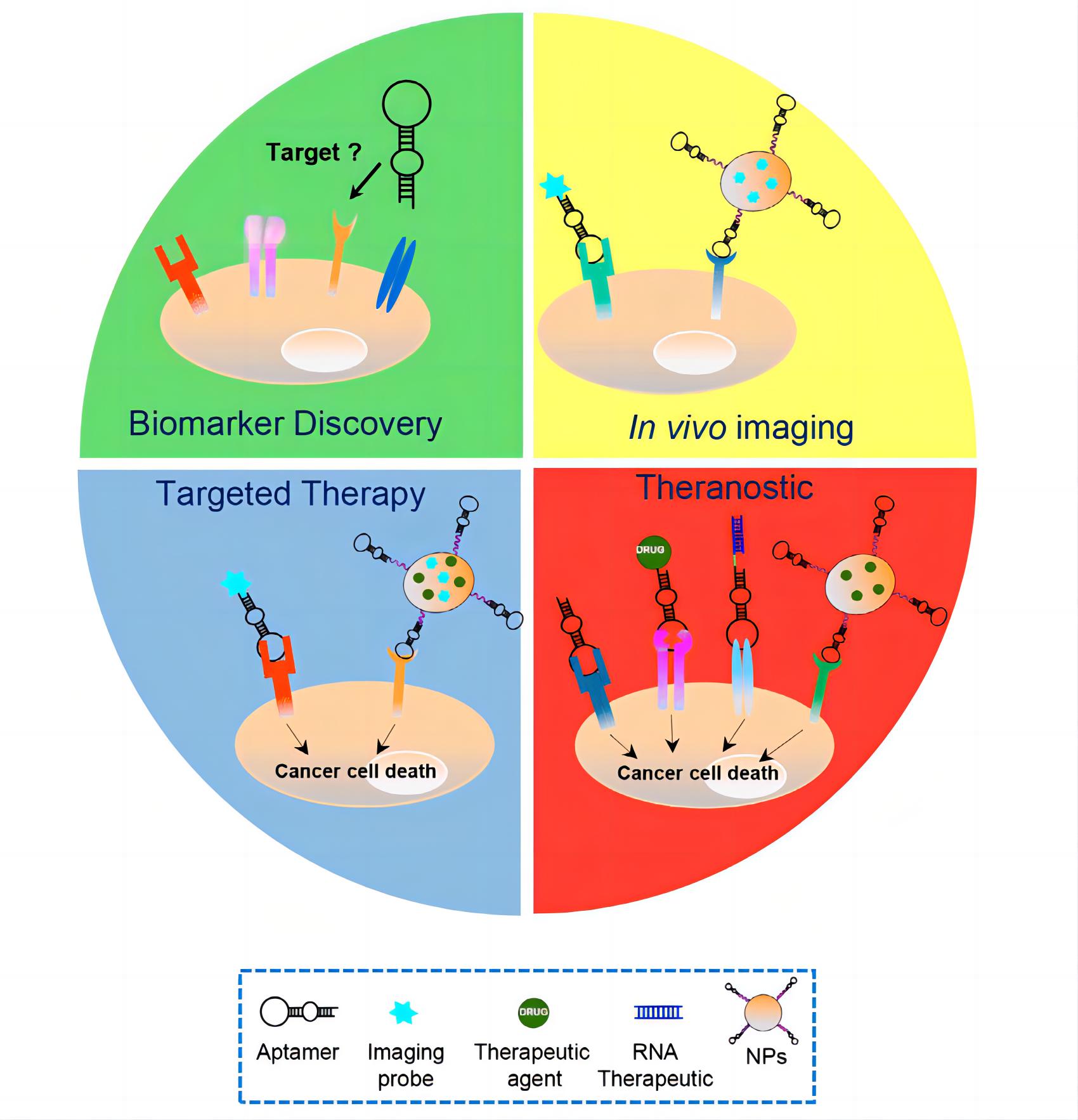 Fig.1 Harnessing aptamers for cancer therapy1, 3
Fig.1 Harnessing aptamers for cancer therapy1, 3
- Detection of Cancer Cells Using Aptamers
CTCs are important for early cancer diagnosis, monitoring of treatment effects, and prognosis. Aptamers evolved from both purified target-based SELEX and Cell-SELEX provide a powerful tool for detecting CTCs. Currently, a variety of aptamers targeting tumor cells have been screened by SELEX.
Table 1. Aptamers targeting markers of cancer cells.2, 3
| Aptamers | Targets | Target Cells |
| SYL3C | EpCAM | MCF7 (breast cancer) |
| A15 | CD133 | Hep3B (liver cancer) |
| Apt928 | CD70 | SKOV-3 (ovarian cancer) |
| A10 | PSMA | LNCaP (prostate cancer) |
| GBI-10 | Tenascin-C | U251 (glioblastoma) |
| TD05 | IGHM | Burkitt's lymphoma |
| Apt1 | CD44 | A549 (lung cancer) |
- Detection of Cancer Tissues Using Aptamers
Currently, IHC is still the gold standard in cancer detection used to confirm the definite diagnosis. However, antibodies used in IHC are diffuse slowly, expensive, and always need signal amplification. In this respect, aptamers have attracted increasing attention as the means to overcome these limitations.
- Cancer Imaging Using Aptamers
By combining fluorescent molecules, radionuclides, and other imaging molecules, aptamers are becoming an important tool for cancer diagnosis. In vivo imaging technologies for tumor diagnosis include CT, MRI, radionuclide-based positron emission tomography (PET), single-photon emission computed tomography (SPECT), and luminescence imaging. For instance, IRD800CW labeled CD30 aptamer is used for in vivo imaging of lymphoma, and Cy5 labeled pancreatic cancer-specific aptamer is used for in vivo imaging of pancreatic cancer.
Services at Creative Biolabs
Creative Biolabs can develop highly-selective aptamers for biomarkers, CTCs, tissue detection. With enhanced selectivity, improved tissue penetration, reduced immunogenicity, and a multitude of detection options, cancer-specific aptamers offer exciting opportunities in blood-based cancer screening, molecular imaging, and targeted therapeutics. Services offered by Creative Biolabs also include negative selection, aptamer-based precipitation, aptamer-based sensors, and aptamer-based microarrays, allosteric/structure-switching aptamer.
Detection Platforms
- Aptamer-Nanoparticle (Apt-NP) System
- Aptamer-Microfluidic System
- Aptamer-Quantum Dots System
- Molecular beacon Aptamer System
- Fluorescent Aptamer System
Start your next project with Creative Biolabs. Contact us right now for more information.
Published Data
1. Novel High-Affinity RNA Aptamers for Gastrointestinal Cancer Biomarkers
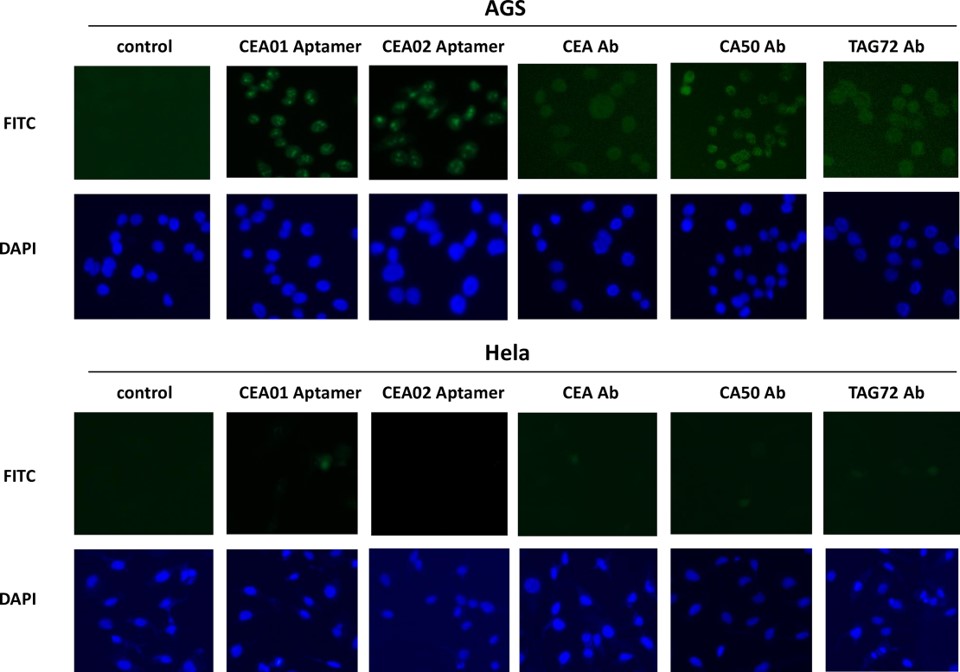 Fig.3 Binding of CEA aptamers to gastric adenocarcinoma cell line AGS.4,3
Fig.3 Binding of CEA aptamers to gastric adenocarcinoma cell line AGS.4,3
In this study, the SELEX technique was used to screen a random 30-mer RNA library for aptamers targeting gastric cancer markers CEA, CA50, and CA72-4. High-throughput sequencing identified six aptamers specific to these biomarkers. Interestingly, the secondary structures of the aptamers showed significant similarity, suggesting structural recognition between the aptamers and the antigens. Dissociation constants were determined using fluorescence spectroscopy, confirming high affinities between the aptamers and their target antigens. Immunostaining of the gastric adenocarcinoma cell line AGS with a CEA aptamer probe showed positive fluorescent signals, indicating its potential for cancer detection. Additionally, transfection of the human colorectal cell line LS-174T with the aptamers resulted in significantly reduced cell viability and growth. These novel RNA aptamers targeting gastrointestinal cancer biomarkers CEA, CA50, and CA72-4 hold promise for improving clinical diagnostics and therapeutic development for gastric cancer.
2. Aptamer-Based Circulating Tumor Cells Detection for Monitoring Breast Cancer Progression
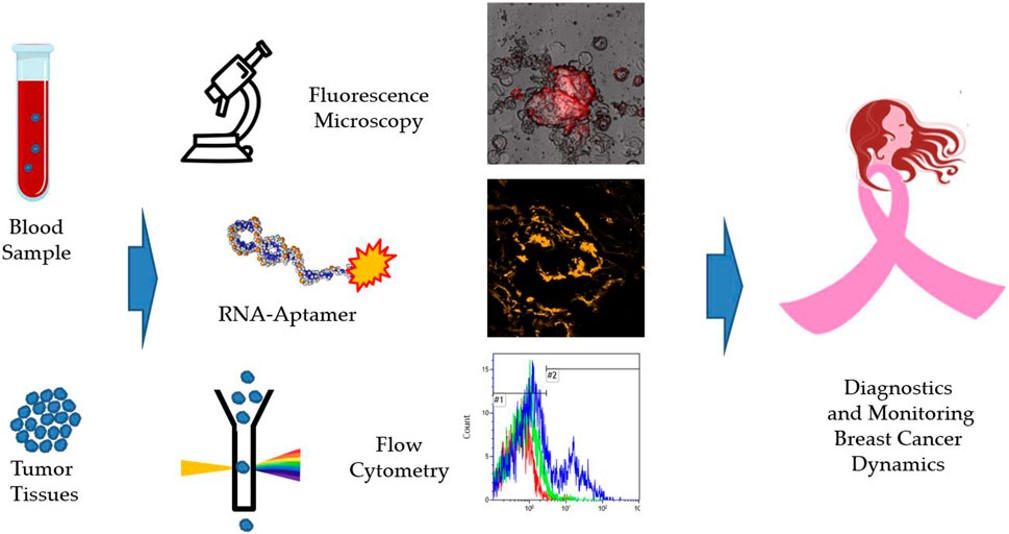 Fig.4 Graphical abstract of this aptamer-based detection method.5,3
Fig.4 Graphical abstract of this aptamer-based detection method.5,3
This study described an RNA aptamer developed for the detection of breast cancer (BC) cells in clinical samples, such as blood. The aptamer was initially chosen through cell-SELEX targeting the MDA-MB-231 cell line. The aptamer showed specificity for oncogenic proteins present on tumor cells in cancer tissues, margins, distant tissues, circulating tumor cells (CTCs) and lymph nodes, in real clinical samples from BC patients of various molecular subtypes. Tissue samples from 27 patients validated the aptamer's ability to bind to various breast cancer subtypes. CTCs were isolated from blood samples of 22 patients before surgery and 7 patients 32 months after surgery using aptamer-based magnetic separation. This non-epithelial aptamer-specific magnetic isolation method offers a simple, minimally invasive diagnostic tool for breast cancer, with isolated intact cells available for molecular diagnostics.
References
- Agnello, Lisa, et al. "Aptamers and antibodies: rivals or allies in cancer targeted therapy?." Exploration of Targeted Anti-tumor Therapy 2.1 (2021): 107.
- Han, Jing, et al. "Application and development of aptamer in cancer: from clinical diagnosis to cancer therapy." Journal of Cancer 11.23 (2020): 6902.
- Distributed under Open Access license CC BY 4.0, without modification.
- Pan, Qing, et al. "Novel RNA aptamers targeting gastrointestinal cancer biomarkers CEA, CA50 and CA72-4 with superior affinity and specificity." PLoS One 13.10 (2018): e0198980.
- Kolovskaya, Olga S., et al. "Monitoring of breast cancer progression via aptamer-based detection of circulating tumor cells in clinical blood samples." Frontiers in Molecular Biosciences 10 (2023): 1184285.
For Research Use Only.