Our kits are crucial for precise cancer diagnosis, enabling the classification of tumors, determination of metastatic origin, and detection of micrometastasis. They also provide vital prognostic and predictive information, such as HER2 status or PD-L1 expression, which are essential for guiding personalized cancer therapies.
In vitro diagnostics (IVD) are pivotal for precise disease detection, diagnosis, and health management. Creative Biolabs stands as a leading expert in IVD development, specializing in the manufacturing of high-quality immunohistochemistry-based kits tailored to meet the specific and diverse project needs of researchers and clinicians, ensuring accurate and reliable results.
Immunohistochemistry
Immunohistochemistry (IHC) is a powerful and indispensable technique that leverages the highly specific interaction between antibodies and antigens to visualize and localize specific proteins within tissue sections. This method combines the precision of immunology with the detailed insights of histology, allowing for the observation of the spatial distribution and expression levels of target molecules directly within their cellular and tissue context.
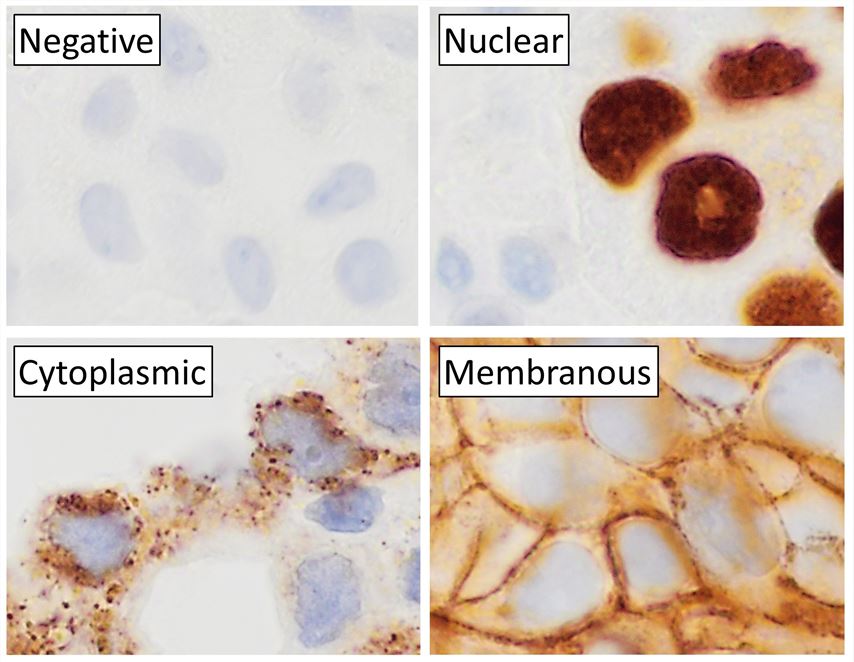 Fig.1 Immunohistochemistry staining.Distributed under CC0 1.0, from Wiki, without modification.
Fig.1 Immunohistochemistry staining.Distributed under CC0 1.0, from Wiki, without modification.
The fundamental workflow of IHC involves several critical steps, beginning with meticulous sample preparation, which includes tissue fixation and sectioning to preserve cellular morphology and antigenicity. This is followed by antigen retrieval, a crucial process that unmasks epitopes often hidden by fixation. Subsequent steps include the application of primary and secondary antibodies, followed by detection systems (e.g., chromogenic or fluorescent) that produce a visible signal, ultimately revealing the precise location of the target protein under a microscope.
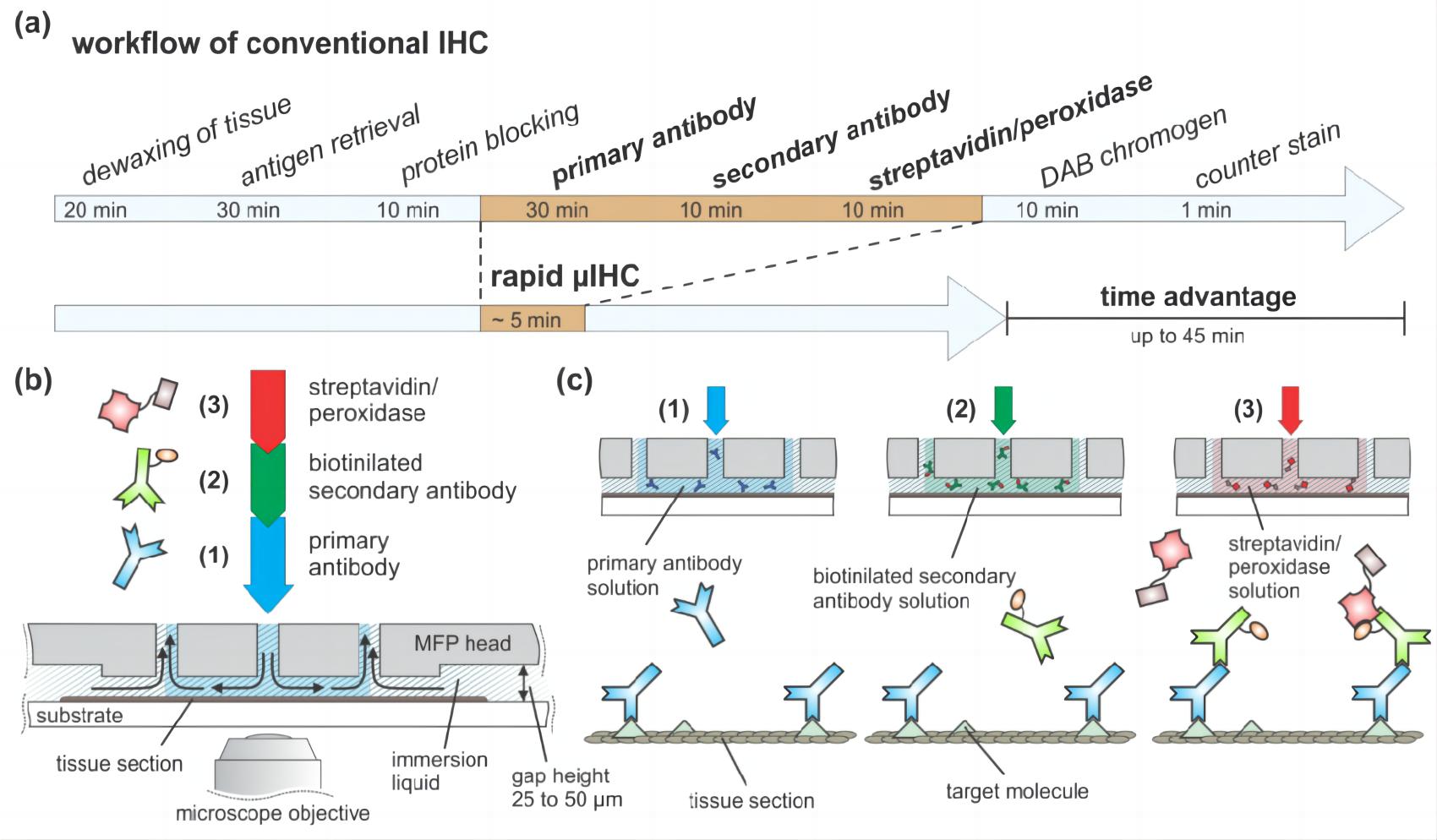 Fig.2 Chromogen-based immunohistochemistry protocol.1,3
Fig.2 Chromogen-based immunohistochemistry protocol.1,3
Advantages of Our IHC Platform
- Exceptional Specificity: IHC relies on highly specific antibody-antigen binding, ensuring that only the target protein is detected, minimizing false positives.
- High Sensitivity: The technique is capable of detecting even low levels of protein expression, providing robust signals for accurate visualization.
- Preservation of Morphology: IHC allows for the visualization of target proteins within the intact cellular and tissue architecture, providing critical contextual information.
- Versatility: It can be applied to a wide range of tissue types and is compatible with various detection systems, offering flexibility for diverse research and diagnostic needs.
- Quantitative Potential: With advanced image analysis, IHC can provide semi-quantitative or quantitative data on protein expression levels and localization.
- Reproducibility: When performed with optimized kits and standardized protocols, IHC delivers consistent and reproducible results, essential for reliable scientific and clinical outcomes.
Our IHC Based Kits Development Services
Creative Biolabs offers bespoke IHC-based kit development services, meticulously designed to provide comprehensive solutions for your specific research and diagnostic needs. Our expertise spans the entire development pipeline, from antibody selection and validation to optimization of detection systems and formulation of all necessary reagents, ensuring each component performs synergistically. We specialize in tailoring kits for various assay formats, including direct, indirect, and advanced multiplex IHC, guaranteeing optimal sensitivity, specificity, and reproducibility for your target antigens and tissue types. Our commitment is to deliver high-performance, ready-to-use kits that streamline your workflow and accelerate your scientific discoveries.
Service Workflow of IHC Based Kits Development
Our IHC-based kit development projects follow a structured and meticulous workflow to ensure optimal results:
The process begins with a detailed discussion to understand your specific research or diagnostic objectives, target antigens, desired assay sensitivity, and tissue types. This initial consultation allows us to tailor the development plan precisely to your requirements.
We meticulously identify high-quality primary and secondary antibodies. Each antibody undergoes extensive validation for specificity, affinity, and performance across relevant tissue panels and various fixation methods to ensure reliable target recognition.
Our expert chemists formulate and optimize all necessary reagents, including antigen retrieval solutions (e.g., pH-optimized citrate or EDTA buffers), blocking sera, washing buffers, detection reagents (e.g., HRP conjugates, chromogens like DAB), and counterstains.
A comprehensive IHC staining protocol is developed, detailing every step from tissue preparation to final visualization. This protocol is then empirically optimized through extensive testing to achieve the ideal signal-to-noise ratio and consistent staining.
Every component and the assembled kit undergo stringent multi-stage QC checks, including functional assays, batch-to-batch consistency testing, and stability studies, to guarantee superior performance and reliability.
Detailed, user-friendly protocols, data sheets, and certificates of analysis are prepared. The kits are then manufactured under strict quality management systems, ensuring adherence to the highest industry standards.
The customized IHC kits are delivered, accompanied by our dedicated technical support team, ready to provide ongoing guidance and troubleshooting assistance to ensure your successful application and interpretation of results.
Applications
Cancer Diagnostics
Biomarker Discovery and Validation
We develop kits instrumental in identifying novel disease biomarkers and validating therapeutic targets. These kits facilitate the visualization and quantification of protein expression patterns critical for understanding disease progression and treatment response.
Drug Development and Preclinical Studies
Our IHC kits are essential tools in preclinical drug development, allowing for the assessment of drug efficacy, toxicity profiling, and detailed analysis of drug mechanisms of action. They are also vital for validating the performance and comparability of biosimilar antibodies.
Infectious Disease Diagnosis
By targeting specific microbial antigens, our kits enable the accurate and timely diagnosis of various infectious diseases, including viral, bacterial, and parasitic infections, providing critical information for patient management.
Personalized Medicine
By enabling the precise identification of specific molecular markers on tissue samples, our IHC kits facilitate the development and application of targeted therapies, ultimately enhancing treatment efficacy and improving patient outcomes in the era of personalized medicine.
Published Data
1. Development of Sensitive Immunohistochemical Techniques for Detection of Neurochemical Molecules
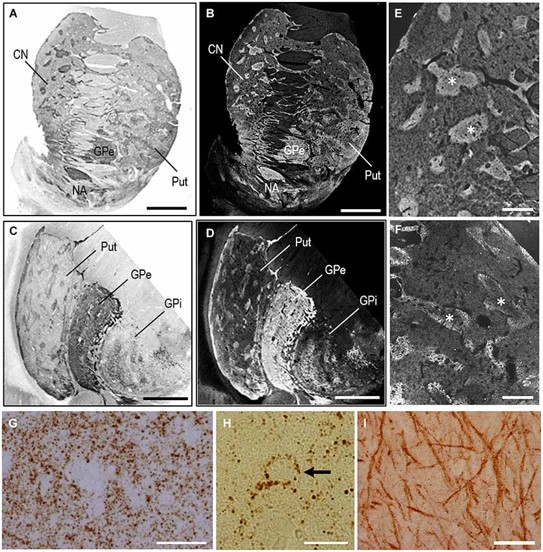 Fig.3 PBTA-DAB staining locates MEnk in the human basal ganglia.2,3
Fig.3 PBTA-DAB staining locates MEnk in the human basal ganglia.2,3
This study presented a highly sensitive IHC method for detecting neurochemical antigens. This IHC method, termed the PBTA method, integrated components of both the polymer and avidin-biotin-complex (ABC) techniques, alongside biotin-tyramide amplification. When applied to [Met]-enkephalin (MEnk) as a target antigen, PBTA for IHC exhibited over 100 times greater sensitivity than the polymer and ABC methods. In enzyme-linked immunosorbent assays, it was about 1,000 times more sensitive than the ABC method. The method's efficacy was evaluated by visualizing neurochemical peptides and proteins in formalin-fixed, paraffin-embedded human brain tissue using both chromogenic and fluorescent detection systems. Results showed that under optimal conditions, this IHC offered high sensitivity while minimizing non-specific background, making it an effective tool for detecting and visualizing trace amounts of neurochemical antigens in autopsied human brains.
2. Development of Rapid Micro-Immunohistochemistry
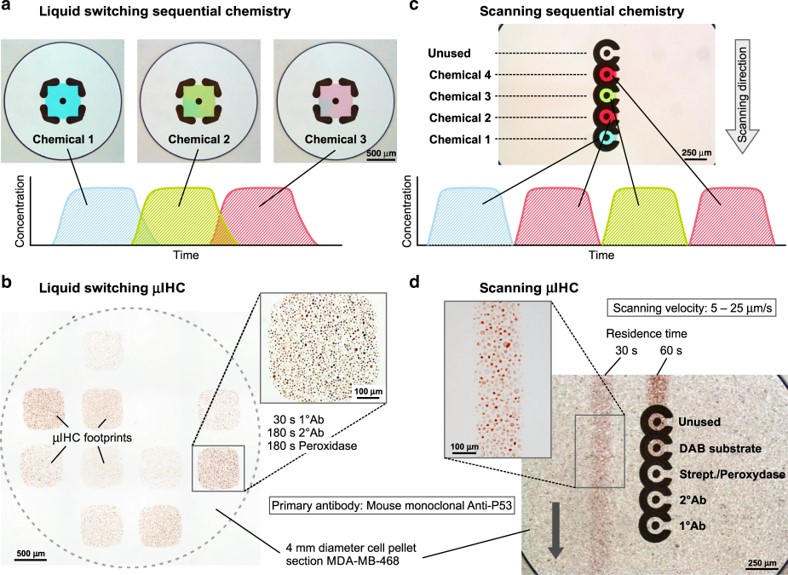 Fig.4 Experimental results of the complete IHC protocol on cell blocks.1,3
Fig.4 Experimental results of the complete IHC protocol on cell blocks.1,3
This study introduced a new approach for rapid and localized immunohistochemical staining of tissue sections using horizontally oriented microfluidic probes (MFPs). These probes featured apertures that created square and circular footprints, allowing for targeted exposure of tissue to time-optimized sequences of biochemicals. The researchers demonstrated that the two main incubation steps in IHC protocols could be completed in under 30 minutes on MDAMB468-1510A cell block sections, significantly reducing the typical incubation time of over an hour in standard methods. This rapid IHC technique could be useful in surgical settings, enabling faster, more dynamic decision-making. Additionally, the method’s efficiency and minimal tissue use make it ideal for multiplexed assays, allowing exploration of optimal conditions with limited tissue while ensuring high-quality staining results for the remainder of the sample.
Service Highlights
- Customization Excellence: We provide highly customized IHC kit development solutions, meticulously tailored to your research or diagnostic project. This ensures optimal performance for your specific target and application.
- Rigorous Quality Control: Our development incorporates multi-stage QC protocols, from raw material inspection to final product validation. This guarantees superior performance, batch consistency, and reliability.
- Expert Scientific Team: Benefit from our expert scientific team, comprising experienced immunologists and biologists. They offer decades of experience in antibody development, assay optimization, and kit manufacturing.
- Comprehensive Support: We offer end-to-end project support, from initial consultation and design to post-delivery technical assistance. This ensures a seamless and successful experience.
- Advanced Technology Integration: Our services leverage cutting-edge technologies, including advanced multiplexing strategies and automated staining platform compatibility. We deliver state-of-the-art IHC solutions for modern laboratories.
FAQs
-
Q: How do you ensure the specificity of the antibodies in your IHC kits?
A: Ensuring antibody specificity is paramount in our development process. We employ extensive screening against a broad panel of tissues and utilize absorption controls, alongside rigorous testing for cross-reactivity, to confirm that antibodies bind exclusively to their intended target antigens.
-
Q: Can your IHC kits be used with automated staining platforms?
A: Yes, our IHC kits are designed with compatibility for automated staining platforms in mind. We optimize our reagents and protocols to ensure seamless integration and consistent performance on various automated systems, enhancing throughput and reproducibility for your laboratory.
-
Q: What is your approach to antigen retrieval optimization in kit development?
A: Our approach to antigen retrieval optimization is highly empirical. We meticulously test and refine various methods, including heat-induced epitope retrieval (HIER) with different pH buffers (e.g., citrate, EDTA) and proteolytic-induced antigen retrieval, to ensure optimal unmasking of epitopes for each specific antibody-antigen pair.
-
Q: How do you address issues of high background staining in your IHC kits?
A: High background staining is meticulously addressed through advanced formulation chemistry and optimized blocking strategies. We develop proprietary blocking reagents and fine-tune washing buffer compositions to minimize non-specific antibody binding, ensuring clear and interpretable staining results.
-
Q: Do your IHC kits include positive and negative control tissues?
A: Absolutely, our IHC kits consistently include expertly prepared positive and negative control tissue slides. These controls are indispensable for validating the assay's performance in every run, allowing users to confirm antibody specificity and protocol efficacy with confidence.
-
Q: How do you ensure batch-to-batch consistency for your IHC kits?
A: Batch-to-batch consistency is guaranteed through our stringent QC program. Every manufactured lot undergoes rigorous functional assays and performance testing, verifying uniform sensitivity, specificity, and staining characteristics across all batches.
-
Q: Can you develop multiplex IHC kits for the simultaneous detection of multiple biomarkers?
A: Yes, we are at the forefront of developing advanced multiplex IHC kits. These innovative solutions enable the simultaneous detection and visualization of multiple biomarkers within a single tissue section, providing richer biological insights and conserving valuable sample material.
Creative Biolabs not only can offer machine-operated IHC staining services, but is skilled at manufacturing IHC-based kits for IVD study. We also provide optimization of experimental conditions for the primary antibody, like concentrations, incubation times, and interpretation of the staining results. There are cell line slides and tissues from different organs can be used for our customers if they do not have. The IHC kits of Creative Biolabs offer qualified reagents and validated tests to perform highly specific immunostaining for further biochemistry research. For more detailed information, please feel free to contact us.
References
- Lovchik, Robert D., David Taylor, and Govind Kaigala. "Rapid micro-immunohistochemistry." Microsystems & Nanoengineering 6.1 (2020): 94.
- Goto, Satoshi, et al. "Development of a highly sensitive immunohistochemical method to detect neurochemical molecules in formalin-fixed and paraffin-embedded tissues from autopsied human brains." Frontiers in neuroanatomy 9 (2015): 22.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.