Repeat Dose Toxicity
Creative Biolabs performs all aspects of non-clinical pharmacokinetic (PK) and toxicokinetics (TK) evaluations in accordance with Good Laboratory Practice (GLP) procedures. We offer repeat dose toxicity studies to give support for clinical studies.
The purpose of repeat dose toxicity test is to characterize the adverse toxicological effects occurring as a result of repeated daily dosing. This study is an integral part of the data package produced to perform quantitative risk assessment (QRA). Our capabilities including the following:
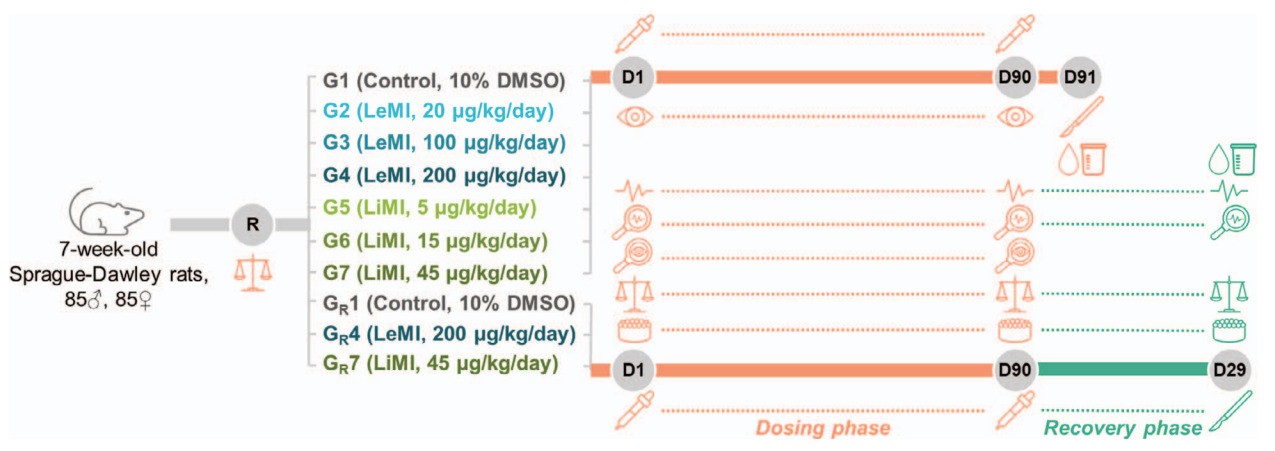 Fig. 1 90-day dose repeat study design. 1
Fig. 1 90-day dose repeat study design. 1
- Toxicity of different organs
- Dose-response (curve)
- Dose selection for subsequent studies
- Margin between toxic and non-toxic dose
- Delayed responses
- Potential accumulation and inhibition
- Metabolites toxicity
- Reversibility/irreversibility effect
- Long-term exposure
- Sex- and species-difference effects
- NOAEL (No Observed Adverse Effect Level)
- NOEL (No Observed Effect Level)
Creative Biolabs offers several types of in vivo repeat dose toxicity assessments, which include:
- 28-day oral toxicity study in rodents
- 90-day oral toxicity study in rodents
- 90-day oral toxicity study in non-rodents
- 21/28-day dermal toxicity study in rodents and non-rodents
- 90-day dermal toxicity study in rodents and non-rodents
- 28-day inhalation toxicity study in rodents
- 90-day inhalation toxicity study in rodents
- Chronic toxicity studies in rodents
Design of Individual Studies
The dose levels, the frequency and administration routes are based on available pharmacodynamic, pharmacokinetic, and toxicological data as well as the intended clinical use. The general design of our repeat dose toxicology studies include:
- Quantification of exposure: The exposure is represented by plasma (or serum or whole blood) concentrations, the AUCs of parent compound and/or metabolite(s).
- Justification of time points for collecting body fluids
- Dose level setting: Low dose levels, intermediate dose levels or high dose levels.
- Determination of the number of tested animals: Performed either in all or a representative proportion, both male and female animals.
- Determination of route of administration
- Metabolites determination
- Statistical evaluation of data:
- Appearance, behavior, ambulation, cognition
- Growth, food consumption, neurological exam, water consumption
- Ophthalmoscopy, body temperature, blood pressure, and ECGs
Analytical Methods
We use various analytical methods to evaluate plasma (or whole blood or serum) concentrations of pharmaceuticals with adequate sensitivity and precision. Our validated analytical methods conform to Good Laboratory Practice (GLP). Methods used in such studies include:
- Gas chromatography
- HPLC
- LC
- LC-MS
- LC-MS-MS
- Capillary electrophoresis
Available Species
- Mice
- Rats
- Rabbits
- Guinea pigs
- Dogs
- Mini pigs
- Non-human primates
Creative Biolabs evaluates and reports all the data within a project in the same consistent way. With our extensive experience in toxicity study, we will provide you the best service of the repeated toxicity study.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
- Agarwal, Anju et al. "Preclinical toxicological assessment of levothyroxine and liothyronine Maillard impurities." Toxicology research vol. 11,5 743-749. 13 Aug. 2022, doi:10.1093/toxres/tfac047. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
