Gram-Negative Bacteria-derived Exosome Research & Applications
Research Highlights OMV Features Why Us Testimonials FAQs
Gram-negative bacteria are widely recognized for their ability to release nanoscale extracellular vesicles, commonly referred to as outer membrane vesicles (OMVs). These exosome-like particles play a central role in microbial ecology, host–microbe interactions, and bacterial communication systems. Unlike Gram-positive vesicles, Gram-negative OMVs are enriched with lipopolysaccharides, phospholipids, and outer membrane proteins, which give them distinctive structural and immunological properties.
In recent years, academic interest in Gram-negative bacterial vesicles has grown rapidly. These vesicles have been shown to facilitate the transport of proteins, nucleic acids, and signaling molecules, thereby shaping immune responses, influencing microbial competition, and mediating cross-kingdom communication. By investigating OMVs, researchers are uncovering new insights into bacterial survival strategies and host adaptation.
Creative Biolabs has developed a comprehensive portfolio of research services dedicated to the study of Gram-negative bacteria-derived exosomes. Our services are designed to help researchers decode their complex biological functions while providing flexible workflows tailored to project-specific needs. By partnering with Creative Biolabs, investigators gain access to highly reproducible vesicle isolation methods, optional downstream characterization, and expert support in experimental design.
Research Highlights Across Gram-Negative Bacterial Exosomes
Research on Gram-negative bacterial vesicles has expanded across diverse species, revealing a range of biological functions and potential utilities. Below is a summary of recent findings reported by the scientific community, illustrating the diversity of Gram-negative OMV activity.
|
Type
|
Reported Findings
|
|
Bacteroides vulgatus-derived Exosome
|
Experimental analysis, including MHC-II expression profiling, synergistic protein detection, and inflammatory cytokine assays in immune cells, demonstrated that Bacteroides vulgatus-derived exosomes containing ligands for TLR4 and TLR2 were capable of inducing a state of immune silencing. This functional property highlights the potential of these vesicles as biological tools for modulating excessive immune activity and as candidates for applications aimed at controlling inflammatory disorders.
|
|
Moraxella catarrhalis-derived Exosome
|
Through time-kill assays, it was revealed that exosomes released by Moraxella catarrhalis enhanced the antimicrobial drug activity against yeast, thereby favoring microbial growth control. In addition, these exosomes were shown to trigger a strong host inflammatory response by activating the human beta-defensin-2 promoter under the regulation of IL-1β. This process resulted in the chemotaxis of neutrophils to the respiratory system and stimulated the release of neutrophil granules enriched with inflammatory mediators.
|
|
Haemophilus influenzae-derived Exosome
|
Exosomes derived from Haemophilus influenzae were demonstrated to act as carriers of suitable adjuvants, functioning as potential vaccine candidates capable of stimulating robust immune responses. In animal studies, mice immunized with these vesicles exhibited high levels of antibody production. Upon subsequent exposure to antigenic challenge, the immunized mice secreted both pro-inflammatory cytokines, such as IFN-γ, and anti-inflammatory regulatory factors, indicating that these vesicles contribute to a balanced and effective immune response.
|
|
Acinetobacter baumannii-derived Exosome
|
Investigations revealed that Acinetobacter baumannii-derived exosomes induced a strong humoral immune response in mice infected with relevant antigens. Notably, intranasal administration of these vesicles promoted the production of secretory IgA in mucosal tissues, which contributed to enhanced mucosal immunity and reduced bacterial transmission. Compared to subcutaneous or intramuscular administration, intranasal delivery was associated with superior protective effects, emphasizing the significance of the administration route in optimizing immunological outcomes.
|
|
Helicobacter pylori-derived Exosome
|
Helicobacter pylori-derived exosomes were found to possess the ability to invade gastric epithelial cells, thereby directly participating in the infection process and mediating bacterial pathogenesis. A key factor contributing to their pathogenicity is the presence of the CagA protein, which plays a central role in toxin-related activity within host cells. Moreover, these vesicles contain multiple immunologically active protein components, including Hop family outer membrane proteins, urease subunit B, and vacuolating cytotoxin-associated proteins. Animal model studies further demonstrated their dual capacity not only to facilitate infection but also to prevent and control H. pylori colonization under certain immunological conditions.
|
|
Porphyromonas gingivalis-derived Exosome
|
Exosomes secreted by Porphyromonas gingivalis were identified as capable of inducing gingipain-dependent cellular damage in both oral epithelial cells and gingival fibroblasts. These vesicles were also found to contain a broad spectrum of bacterial adhesion molecules, such as protofibrillar proteins, gingipains, and hemagglutinins. The presence of these adhesion factors supports inter-bacterial communication within oral microbial communities, promotes plaque diversity, and contributes to the development and progression of periodontitis-associated diseases.
|
|
Bordetella pertussis-derived Exosome
|
Exosome-like vesicles isolated from Bordetella pertussis were encapsulated into sodium alginate nanoparticles to construct a sustained-release drug delivery system. In murine models, treatment with this vesicle-based drug system was associated with elevated immune indices and enhanced secretion of immune mediators, including antibodies, IL-17, and IL-10. These findings underline the potential utility of such engineered vesicles in developing novel immunomodulatory platforms.
|
|
Burkholderia thailandensis-derived Exosome
|
Studies have shown that exosomes released by Burkholderia thailandensis provide significant protective effects against melioidosis pneumonia in animal models by activating innate immune pathways. These vesicles were also found to encapsulate a variety of antimicrobial compounds, such as rhamnolipids and peptidoglycan hydrolases, which reinforce their protective properties and highlight their multifunctional role in both antimicrobial defense and host-pathogen interactions.
|
|
Pseudomonas aeruginosa-derived Exosome
|
Experimental assays using corneal epithelial cells and neutrophils demonstrated a dual role for Pseudomonas aeruginosa-derived exosomes. On one hand, they promoted bacterial survival by inducing the release of pro-inflammatory cytokines in host cells. On the other hand, they facilitated neutrophil chemotaxis and subsequent respiratory bursts, processes that, while enhancing host defense, may also contribute to collateral tissue damage and the persistence of infection.
|
|
Klebsiella pneumonia-derived Exosome
|
Exosomes from Klebsiella pneumoniae were investigated in tumor cell and hormonal mouse models, where they were found to encapsulate chemotherapeutic agents. These vesicles not only improved the pharmacokinetics and delivery efficiency of chemotherapeutic drugs but also amplified their therapeutic efficacy by recruiting macrophages to accumulate in tumor tissues. This synergistic effect arose from the inherent immunogenicity of the vesicles, which acted to enhance the overall antitumor immune response.
|
|
Escherichia coli-derived Exosome
|
Escherichia coli-derived exosomes were observed to inhibit the fusion of autophagosomes with lysosomes, thereby impairing autophagic clearance mechanisms within host cells. Additionally, these vesicles were shown to activate the noncanonical inflammasome pathway in a dose-dependent manner, a process dependent on the expression of the virulence factor HlyF by the parent bacterium. In contrast, exosomes obtained from E. coli mutants lacking functional HlyF failed to induce this autophagy blockade, underscoring the critical role of HlyF in vesicle-mediated pathogenic mechanisms.
|
|
Salmonella typhimurium-derived Exosome
|
Exosomes from Salmonella typhimurium were engineered to carry recombinant receptor-binding domains against a specific virus. In vaccinated hamster models, these vesicles successfully induced both plasma and mucosal antibody responses. Upon subsequent viral challenge, the immunized animals were protected, as evidenced by the production of virus-neutralizing antibodies that significantly alleviated disease pathology. This finding highlights the promising role of engineered bacterial vesicles in the development of next-generation vaccine platforms.
|
|
Neisseria meningitidis-derived Exosome
|
Exosomes isolated from a genetically modified strain of Neisseria meningitidis deficient in major outer membrane proteins were tested in mouse models of lower genital tract infection. Immunization with these vesicles led to the generation of specific antibodies capable of recognizing a broad range of gonococcal antigens. The resulting immune response conferred both faster and stronger resistance against gonococcal challenge, suggesting their potential in the design of improved vaccines against sexually transmitted bacterial pathogens.
|
|
Neisseria lactamica-derived Exosome
|
Researchers investigated a strategy to biotinylate Neisseria lactamica-derived exosomes and subsequently couple target antigens onto their surface via the high-affinity binding between rhizavidin and biotin. The engineered vesicles demonstrated efficient antigen presentation, significantly enhanced antigen recognition by the host immune system, and triggered stronger immune responses compared to unmodified exosomes. These results emphasize the potential of N. lactamica vesicles as versatile antigen delivery platforms.
|
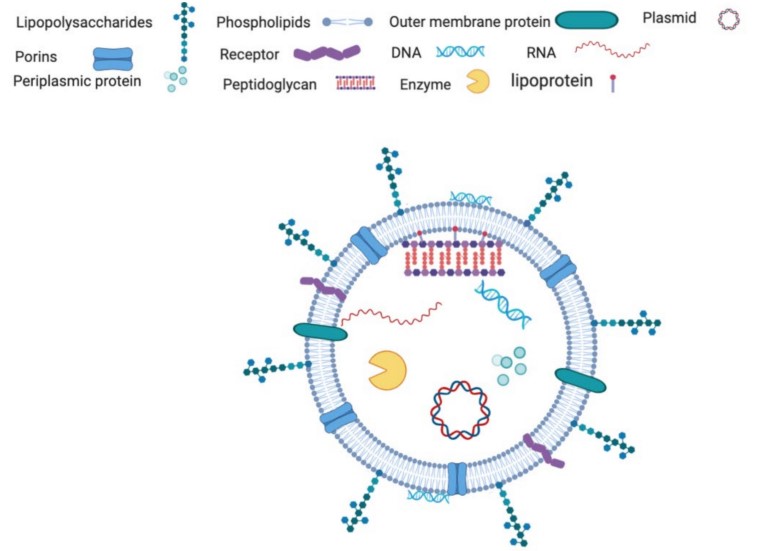 Fig.1 Common Components of Vesicles Derived from Gram-Negative Bacteria.1
Fig.1 Common Components of Vesicles Derived from Gram-Negative Bacteria.1
Unique Features of Gram-Negative OMVs
Structural composition
Cargo protection
Immunogenicity
Functional plasticity
Structural composition
Contain phospholipids, lipopolysaccharides (LPS), outer membrane proteins, and periplasmic components.
Cargo protection
Capable of long-distance transport of proteins, nucleic acids, and signaling molecules while shielding cargo from degradation.
Immunogenicity
Due to LPS and outer membrane proteins, OMVs are potent immune modulators.
Functional plasticity
Serve as mediators of host–pathogen interactions, nutrient cycling, and microbial adaptation in various niches.
Creative Biolabs integrates these biological insights into tailored research workflows, enabling clients to study OMVs in a structured and scientifically rigorous manner.
Why Choose Creative Biolabs?

Proven expertise
Years of accumulated experience in bacterial exosome research.

Reliable infrastructure
Advanced ultracentrifuges, chromatography systems, and electron microscopy facilities.

Flexible services
From basic vesicle preparation to full-scale functional profiling.

Scientific integrity
Clear distinction between standard workflows and optional services ensures transparency.
Testimonials from Collaborators
"Working with Creative Biolabs allowed us to accelerate our Gram-negative vesicle project significantly. Their isolation workflow was both robust and flexible, and their technical support was exceptional."
– Research Scientist, USA
"The optional proteomics and imaging analyses offered by Creative Biolabs gave us data we would not have been able to generate in-house. Their team understood our project needs and provided tailored solutions."
– Research Scientist, Italy
Gram-negative bacterial exosomes represent a rapidly expanding field of research with broad implications for microbiology, immunology, and biotechnology. By providing rigorous, customizable workflows and advanced analytical options, Creative Biolabs supports researchers in unlocking the complex biology of these vesicles. With Creative Biolabs as a partner, clients can confidently pursue projects ranging from basic exosome isolation to detailed functional studies, all supported by a platform built on expertise, precision, and scientific reliability. Feel free to reach out.
FAQs
Q: What are the functional roles of exosomes derived from Gram-negative bacteria?
A: Exosomes from Gram-negative bacteria play crucial roles in mediating intercellular communication, facilitating the transfer of biomolecules between cells. They are involved in processes like biofilm formation, stress response, and evasion of host immune responses. Their ability to influence recipient cells highlights their importance in microbial ecology and pathogenesis.
Q: How can Gram-negative bacterial exosomes be utilized in research?
A: Studying Gram-negative bacterial exosomes can reveal insights into bacterial interactions, pathogenic mechanisms, and host response. They can serve as models for understanding microbial communication and may be utilized in developing new biotechnological applications, such as drug delivery systems, biosensors, or environmental remediation strategies.
Q: What are some potential applications of Gram-negative bacterial exosomes?
A: Gram-negative bacterial exosomes have several potential applications, including synthetic biology, where they can be used to construct delivery vehicles for genetic materials, develop biopesticides, and study microbial ecology. Additionally, their components may be harnessed for biomarker discovery or to create novel bioproducts in industrial microbiology.
Q: How do Gram-negative bacterial exosomes differ from those derived from other organisms?
A: Gram-negative bacterial exosomes often contain unique lipopolysaccharides and protein markers that distinguish them from eukaryotic exosomes. Their biogenesis and composition can vary significantly due to differences in cell wall structures and mechanisms of intercellular communication, reflecting the distinct physiological contexts of bacteria compared to other organisms.
Reference
-
Dell'Annunziata, Federica et al. "Gene Transfer Potential of Outer Membrane Vesicles of Gram-Negative Bacteria." International journal of molecular sciences vol. 22,11 5985. Distributed under Open Access license CC BY 4.0. The image was modified by revising the title. https://doi.org/10.3390/ijms22115985
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Common Components of Vesicles Derived from Gram-Negative Bacteria.1
Fig.1 Common Components of Vesicles Derived from Gram-Negative Bacteria.1













