Salmonella typhimurium-derived Exosome Research & Application
Overview Workflow Insights Our Advantages Client Perspectives FAQs
Understanding bacterial extracellular vesicles has opened new perspectives in microbiology and immunology. Among these, Salmonella typhimurium-derived exosomes stand out for their multifaceted role in bacterial communication and host immune modulation.
At Creative Biolabs, we dedicate our expertise to advancing exosome research by supporting fundamental studies on Gram-negative bacteria, helping scientists decode vesicle-mediated molecular interactions, signaling pathways, and functional implications across biological systems.
Scientific Overview
Biological Origin and Significance
Salmonella typhimurium, a facultative intracellular pathogen, naturally releases nanoscale vesicles that mirror the complexity of bacterial physiology. These vesicles, often referred to as outer membrane vesicles (OMVs), encapsulate proteins, lipids, nucleic acids, and virulence-related molecules.
In recent studies, such vesicles have been engineered or adapted to investigate host immune activation, intracellular signaling, and bacterial survival mechanisms.
Host Interaction and Immune Activation
S. typhimurium-derived exosomes can interact with immune and epithelial cells, stimulating cytokine secretion and antigen presentation. Their molecular contents allow researchers to examine how bacteria modulate inflammation and autophagy, and how vesicle components affect mucosal defense.
Emerging Research Utility
Scientists have begun exploring the use of these vesicles as natural nanocarriers for antigen presentation in vaccine development and as experimental tools for studying vesicle trafficking in host-pathogen systems.
Expanding Research Landscape
The growing interest in Salmonella-derived vesicles highlights their potential as model systems for understanding Gram-negative bacterial communication and immune crosstalk — areas where Creative Biolabs provides both the tools and analytical support for next-generation exosome studies.
Connect with Creative Biolabs' experts to learn how our tailored exosome solutions can empower your bacterial research.
Isolation and Custom Workflow
At Creative Biolabs, the standard workflow for isolating Salmonella typhimurium-derived exosomes is designed to ensure high purity, reproducibility, and integrity of bacterial vesicles. Each stage is optimized to minimize contamination and preserve biological activity. The standard process focuses on obtaining pure vesicles for research use, while additional analyses (Protein or RNA content profiling) can be included upon request depending on species-specific data and strain library availability.
1.Bacterial Culture Preparation
-
Grow Salmonella typhimurium in LB liquid medium under constant shaking (180–200 rpm) to maintain aerobic conditions.
-
Monitor growth using spectrophotometry until OD600 ≈ 1.0, corresponding to the logarithmic growth phase when vesicle secretion is most active.
-
Ensure consistent temperature control (typically 37 °C) and avoid overgrowth, which may alter vesicle composition or yield.Primary Centrifugation
2.Primary Centrifugation
-
Centrifuge the bacterial culture at low speed (e.g., 10,000 × g for 15 min at 4 °C) to remove intact cells and large debris.
-
Carefully transfer the supernatant without disturbing the pellet to prevent contamination by cell fragments.
-
Maintain samples on ice throughout to prevent vesicle degradation and protein denaturation.
3.Supernatant Filtration
-
Pass the supernatant through a 0.22 μm sterile membrane to remove any remaining bacterial cells.
-
Use low-pressure filtration systems to minimize vesicle deformation or loss.
4.Ultrafiltration & Concentration
-
Employ tangential flow filtration (TFF) or centrifugal filter devices with appropriate molecular weight cut-offs to concentrate the vesicle-rich fraction.
-
Control transmembrane pressure and flow rate to avoid shear stress that could disrupt vesicle membranes.
-
Optionally, buffer-exchange the concentrated sample into PBS to prepare for subsequent ultracentrifugation.
5.Ultracentrifugation
-
Subject the concentrated supernatant to high-speed ultracentrifugation to pellet the Salmonella typhimurium-derived exosomes.
-
Wash the pellet once or twice in sterile PBS to remove residual media components and non-vesicular proteins.
-
Resuspend gently to maintain vesicle integrity and avoid aggregation.
6.Resuspension and Storage
-
Resuspend purified vesicles in sterile, filtered PBS at the desired concentration for downstream use.
-
Aliquot samples to prevent repeated freeze-thaw cycles that may compromise vesicle stability.
-
Store at –80 °C for long-term preservation or at 4 °C for short-term experiments (within 48 hours).
To design your own Salmonella exosome isolation plan, reach out to Creative Biolabs' technical support specialists.
Scientific Insights: Features of Salmonella typhimurium-Derived Exosomes
|
Research Focus
|
Key Findings from Literature
|
|
Molecular Modification of Vesicles
|
Recombinant fusion proteins containing SpyTag and viral receptor-binding domains (RBDs) were successfully conjugated to Salmonella typhimurium-derived exosomes using SpyCatcher technology, demonstrating the feasibility of functionalized bacterial exosomes.
|
|
Evaluation of Safety in Animal Models
|
Intranasal immunization with modified exosomes in hamsters caused no adverse effects on body weight or temperature, confirming the safety of the vesicle-based antigen delivery system.
|
|
Antibody Induction and Immune Response
|
Repeated administration led to significant increases in serum IgG titers targeting viral RBDs, suggesting that Salmonella-derived exosomes effectively stimulate systemic immunity.
|
|
Mucosal Immunity Activation
|
Elevated IgA and IgM levels were detected in bronchoalveolar lavage samples of immunized animals, indicating successful mucosal immune engagement—an important feature for respiratory infection studies.
|
|
Reduction of Viral Load and Tissue Damage
|
Post-challenge analyses revealed decreased viral titers and alleviated lung inflammation in vesicle-immunized animals, confirming that these exosomes mediate protective immune modulation.
|
Contact Creative Biolabs to explore how these findings can guide your next experimental design.
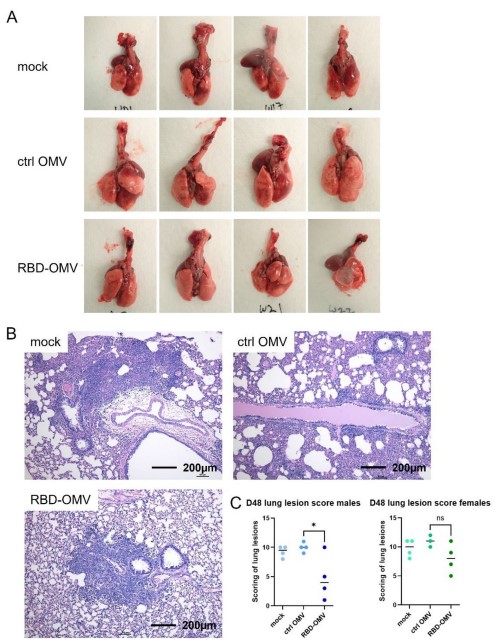 Fig.1 Modified Salmonella typhimurium exosome vaccine significantly alleviated lung lesions in virus-attacked hamsters.1
Fig.1 Modified Salmonella typhimurium exosome vaccine significantly alleviated lung lesions in virus-attacked hamsters.1
Key Advantages of Partnering with Creative Biolabs
 Comprehensive Expertise in Bacterial Vesicle Research
Comprehensive Expertise in Bacterial Vesicle Research
Creative Biolabs integrates microbiology, proteomics, and vesicle analytics to deliver consistent and high-quality research data for Gram-negative exosomes.
 Tailored Study Design
Tailored Study Design
Flexible project models allow clients to choose between exploratory isolation, molecular characterization, or targeted vesicle engineering.
 Strict Quality and Reproducibility Standards
Strict Quality and Reproducibility Standards
All projects adhere to controlled conditions to ensure batch consistency and data reliability across biological replicates.
 Scalable Experimental Support
Scalable Experimental Support
From pilot investigations to multi-sample comparative studies, Creative Biolabs supports scalable workflows with transparent cost structures.
Collaborate with Creative Biolabs to experience research precision built on trust, reproducibility, and innovation.
Customer Impressions
Salmonella-derived vesicle research represents a rapidly growing field bridging microbiology, nanobiology, and immunology. By supporting global research teams, Creative Biolabs continues to empower innovative projects aimed at dissecting vesicle-mediated mechanisms of host interaction, immune regulation, and molecular signaling. Researchers who have collaborated with Creative Biolabs consistently emphasize:
"The precision and transparency of Creative Biolabs' vesicle preparation pipeline exceeded our expectations — particularly in reproducibility across replicates."
"Their technical team provided timely updates and shared practical insights on optimizing Salmonella vesicle yields. It felt like a real scientific partnership."
"Creative Biolabs' analytical reports were comprehensive and publication-ready, which greatly accelerated our manuscript preparation process."
Contact us to learn how Creative Biolabs drives precision exosome research.
Salmonella typhimurium-derived exosomes offer an exceptional platform for studying bacterial-host interaction, immune modulation, and nanovesicle biology. With Creative Biolabs' customizable workflows, optional analytical add-ons, and data-driven research infrastructure, scientists gain a trusted partner in the exploration of Gram-negative bacterial vesicles. Start your Salmonella vesicle research with Creative Biolabs today - inquire about customized solutions and pilot studies.
FAQs
Q: What are exosomes derived from Salmonella typhimurium, and how do they differ from exosomes derived from other sources?
A: Exosomes derived from Salmonella typhimurium are small extracellular vesicles secreted by the bacterium during its lifecycle. These exosomes differ from those derived from mammalian cells due to their origin. Their distinct molecular signatures can provide insights into bacterial-host interactions and immune modulation.
Q: What role do Salmonella typhimurium exosomes play in bacterial pathogenesis?
A: Salmonella typhimurium exosomes play a crucial role in pathogenesis by facilitating intercellular communication, evading the host immune response, and promoting bacterial survival in hostile environments. They contain virulence factors that can alter host cell functions, modulate immune responses, and enhance bacterial invasion, supporting the bacterium's persistence and pathogenicity.
Q: How can Salmonella typhimurium-derived exosomes be used in research settings?
A: In research, Salmonella typhimurium-derived exosomes can be utilized as tools for studying bacterial communication and host-pathogen interactions. They can serve as models for investigating exosomal biology and platforms for delivering genetic material or antigens in vaccine development. Additionally, researchers can analyze the content and effects of these exosomes to gain insights into bacterial behavior and immune evasion strategies.
Q: What is the potential application of Salmonella typhimurium-derived exosomes in biotechnology?
A: The biotechnological applications of Salmonella typhimurium-derived exosomes include their use as novel drug delivery systems or as vehicles for targeted therapies. Due to their ability to navigate biological barriers and their inherent stability, these exosomes could facilitate the delivery of therapeutic agents to specific cell types, enhancing the efficacy of treatments, particularly in fields such as immunology and microbiology.
Q: Are there any studies that highlight the unique cargo of Salmonella typhimurium exosomes?
A: Yes, several studies have identified unique proteins, lipids, and RNA species within Salmonella typhimurium exosomes that play significant roles in modulating host cell responses. These studies have highlighted the presence of virulence factors and immune-modulatory molecules that can alter host signaling pathways and immune activation, providing a deeper understanding of pathogenic mechanisms.
Q: How do Salmonella typhimurium-derived exosomes affect host immune responses?
A: Salmonella typhimurium-derived exosomes can modulate host immune responses by delivering immunomodulatory molecules that either enhance or suppress immune pathways. They may interfere with antigen presentation, inhibit pro-inflammatory cytokine production, or induce apoptosis in immune cells, thereby enabling the bacterium to evade detection and persist in the host.
Reference
-
Jiang, Linglei, et al. "A bacterial extracellular vesicle‐based intranasal vaccine against SARS‐CoV‐2 protects against disease and elicits neutralizing antibodies to wild‐type and Delta variants." Journal of extracellular vesicles 11.3 (2022): e12192. Distributed under Open Access license CC BY 4.0. The image was modified by revising the title. https://doi.org/10.1002/jev2.12192.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Modified Salmonella typhimurium exosome vaccine significantly alleviated lung lesions in virus-attacked hamsters.1
Fig.1 Modified Salmonella typhimurium exosome vaccine significantly alleviated lung lesions in virus-attacked hamsters.1









