Stem Cell-derived Exosome Application
- Kidney Injury Repair
Overview Services Features FAQs
Creative Biolabs offers exosome research services to help develop the repair potential of SC-Exo (stem cell exosomes) for kidney injury, as recent studies have demonstrated the benefits of SC-Exo in regenerative medicine.
Overview of SC-Exo to Repair Kidney Injury
The use of SC-Exo for the repair of kidney injury has progressed in a variety of cellular and animal models. It will likely be a new strategy for the treatment of kidney disease.
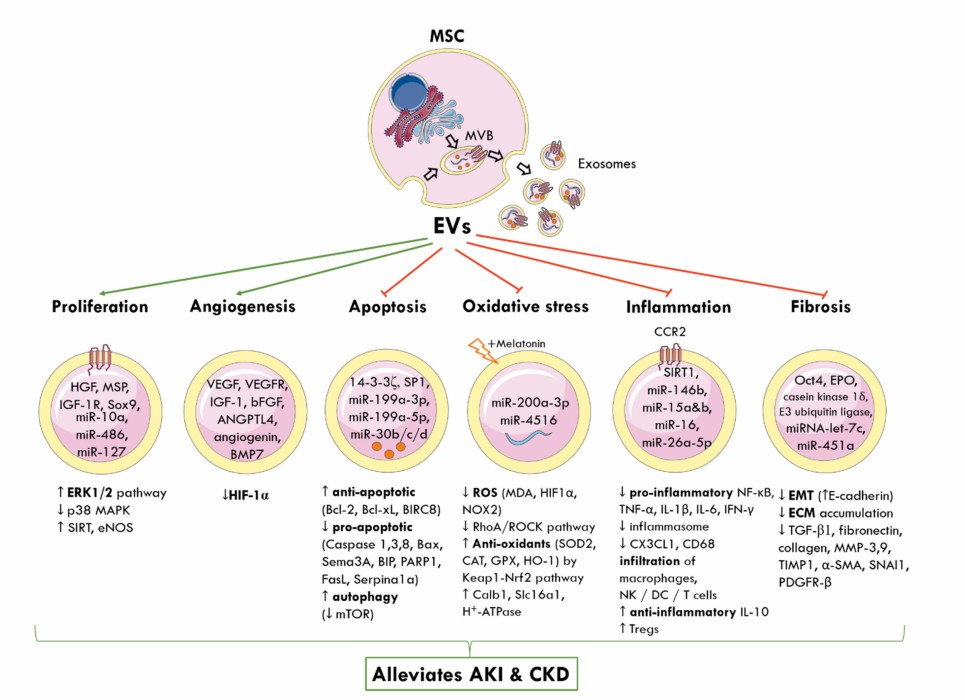 Fig. 1 SC-Exo cause kidney protection in acute kidney injury and chronic kidney disease.1, 3
Fig. 1 SC-Exo cause kidney protection in acute kidney injury and chronic kidney disease.1, 3
|
SC-Exo
|
Application
|
|
Urine-derived SC-Exo
|
Human urine-derived SC-Exo inhibit nuclear translocation of renal NF-κB p65 and infiltration of inflammatory cells in a rat kidney injury model by delivering miR5a-1p targeting IRAK1 to protect renal function and have the advantage of both easy access to materials.
|
|
Umbilical cord mesenchymal SC-Exo
|
Umbilical cord MSC-derived exosomes were shown to efficiently homing in the proximal tubules of ischemic kidneys via surface very late antigen-4 and lymphocyte function-associated antigen 1, inhibiting p53 protein expression by delivering miR125b-5p, thereby attenuating ischemia-reperfusion injury-induced necrotizing apoptosis of renal tubular epithelial cells and promote tubular repair.
|
|
Bone marrow SC-Exo
|
Indoleamine 2,3-dioxygenase overexpression in bone marrow mesenchymal SC-Exo exposure promotes polarization of the anti-inflammatory phenotype of macrophages. It promotes self-repair of ischemic kidney injury in mice by altering the inflammatory microenvironment of renal tubular cells.
|
|
Adipose SC-Exo
|
Adipose SC-Exo promote autophagic flux and reduce podocyte apoptosis by enhancing miR-486 expression, which will thereby attenuate renal injury in diabetes.
|
Our Services
We not only support isolation, exosome characterization, and profiling services related to SC-Exo, but can also assist in the expansion of SC-Exo functions, such as target protein modification and cargo loading, through innovative exosome engineering platforms to assist in obtaining customized exosomes and conducting in-depth studies.
Features and Triggers of Kidney Injury
-
Most kidney injuries occur within the kidney, for example, renal vascular and glomerular vein thrombosis; inflammation of the glomerular inner and outer membranes; hemorrhage of the glomerular inner and outer membranes and basement membrane; ischemia and necrosis of the renal tubules.
-
Kidney injury is often accompanied by lower blood pressure, lower hemoglobin, decreased urine output, and sometimes acute renal failure.
-
When the kidney is directly injured, the red blood cells and blood creatinine rise rapidly due to the obstruction of blood and lymphatic circulation in the glomerulus.
-
The physiopathological characteristics of the kidney are closely related to its pathological changes, and kidney injury from various causes can cause different degrees of renal failure. After kidney injury, fibrosis and sclerosis can form locally, leading to tubular atrophy and loss of function, and eventually developing into end-stage renal disease.
|
Triggers
|
|
Hypoxia
|
Ischemia
|
|
Hypoxia can cause endothelial cell damage, necrosis, and the formation of renal microthrombosis, which can cause vasospasm, thrombosis, increased pressure in the renal ischemic zone, and lead to increased intracapillary pressure in the glomerulus and damage to the glomerulus, and also cause atherosclerosis of the renal arteries and the formation of thrombus.
|
The blood vessels in the kidney are more densely distributed, and ischemia can produce a large number of free radicals, which can damage kidney tissue cells and cause kidney tissue damage. In acute renal failure, tubular necrosis and decreased hematocrit are common pathologic triggers. Tubular necrosis produces large amounts of intracellular and extracellular fluid, which increases tubular pressure and leads to tissue ischemia and hypoxia.
|
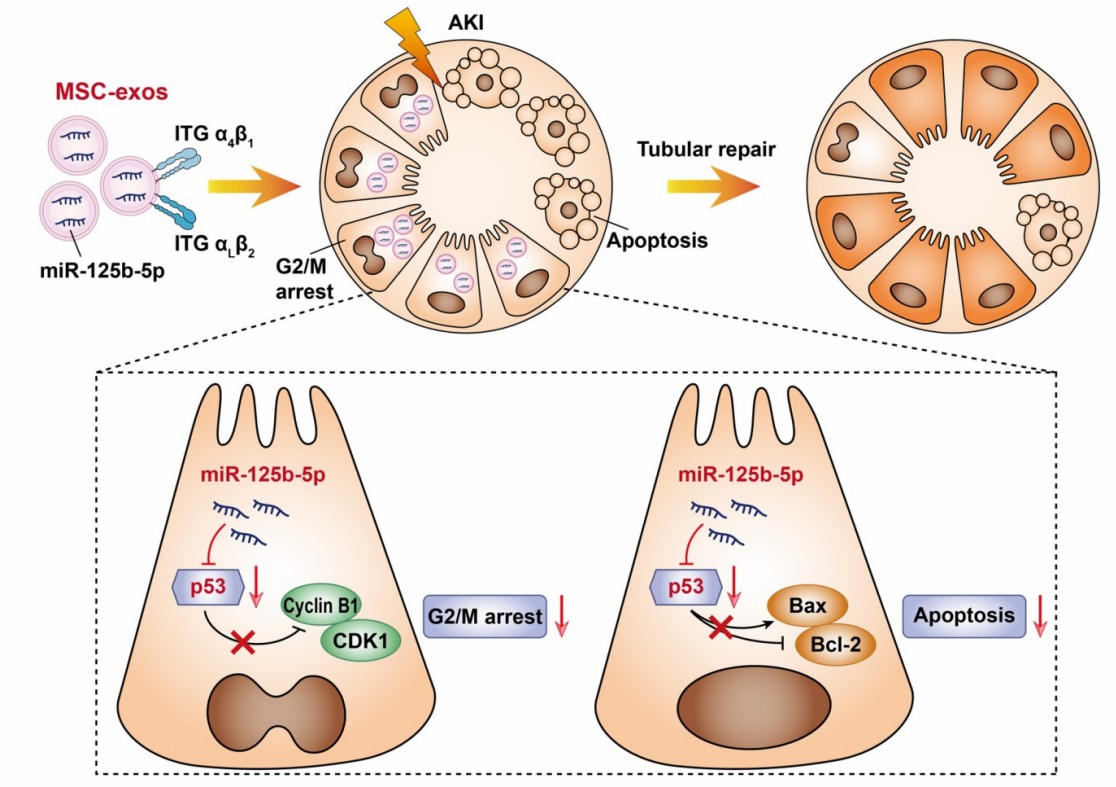 Fig. 2 Mechanism of mesenchymal SC-Exo in the treatment of acute kidney injury. 2, 3
Fig. 2 Mechanism of mesenchymal SC-Exo in the treatment of acute kidney injury. 2, 3
Creative Biolabs supports custom studies of SC-Exo from multiple sources to help clients discover the possibilities of SC-Exo in the repair of kidney injury applications. Please contact us with your request.
FAQs
Q: What are the key mechanisms by which stem cell-derived exosomes promote kidney repair?
A: Stem cell-derived exosomes facilitate kidney repair through several mechanisms, including the transfer of bioactive molecules that modulate inflammation, promote cell survival, and enhance tissue regeneration. They also play a role in the paracrine signaling pathways that activate endogenous repair processes.
Q: How do the properties of exosomes vary depending on the source of stem cells?
A: The properties of exosomes can differ significantly based on the type of stem cells used. These differences can affect the cargo composition influencing their therapeutic efficacy in kidney injury models.
Q: What role do microRNAs in stem cell-derived exosomes play in kidney injury repair?
A: MicroRNAs within stem cell-derived exosomes can regulate gene expression in recipient cells, influencing processes such as apoptosis, inflammation, and fibrosis. Understanding the specific microRNAs involved in kidney repair can provide insights into their therapeutic potential and mechanisms of action.
Q: What are the current models used to study the effects of stem cell-derived exosomes on kidney injury?
A: Various in vitro and in vivo models are employed, including renal tubular epithelial cell cultures, animal models of acute and chronic kidney injury, and organ-on-a-chip systems. Each model provides unique insights into the mechanisms of action and therapeutic efficacy of exosomes.
Q: What future directions are being explored in the research of stem cell-derived exosomes for kidney injury repair?
A: Future research directions include exploring the use of exosomes in combination with other therapeutic modalities, investigating their long-term effects on kidney function, and conducting large-scale studies to assess their safety and efficacy in preclinical models. Additionally, advancements in exosome engineering and delivery systems are being actively pursued.
References
-
Birtwistle, Lucy, Xin-Ming Chen, and Carol Pollock. "Mesenchymal stem cell-derived extracellular vesicles to the rescue of renal injury." International Journal of Molecular Sciences 22.12 (2021): 6596.
-
Cao, Jing-Yuan, et al. "Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury." Theranostics 11.11 (2021): 5248.
-
Under open access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 SC-Exo cause kidney protection in acute kidney injury and chronic kidney disease.1, 3
Fig. 1 SC-Exo cause kidney protection in acute kidney injury and chronic kidney disease.1, 3
 Fig. 2 Mechanism of mesenchymal SC-Exo in the treatment of acute kidney injury. 2, 3
Fig. 2 Mechanism of mesenchymal SC-Exo in the treatment of acute kidney injury. 2, 3









